Introduction
The rhizosphere is an intimate area of soil near the root system of the plant1. The rhizosphere consists of a micro-ecosystem that involves the root system of a plant, microorganisms, nematodes, etc.2. The interaction between microorganisms and plants is special and beneficial to each other. Microbial components consist of bacteria and fungi, having multifarious plant growth-promoting potential such as nutrient acquisition (nitrogen fixation, phosphate/ zinc/ potassium solubilization), Siderophore production, ACC deaminase production, plant growth-promoting hormones(Auxins, Gibberellins)etc.3. IAA is an important auxin that regulates plant growth and development, and also act an important signaling molecule. Ortiz-Castro R et al.(2012)4 and Raheem et al.(2018)5 have reported that IAA-producing bacteria viz, Bacillus amyloliquifacience significantly increased the length of the wheat plant and B. muralis D-5 and Enterobacter aerogenes S-10 had a positive impact on spike length and seed weight under drought stress. IAA-producing plant growth-promoting microorganisms (PGPM) include Pseudomonas, Bacillus, Azotobacter, Acinetobacter, Rhizobium, Bradyrhizobium, Burkholderia, Candida tropicalis, Ustilago esculanta etc.6,7. Cultural conditions for IAA production is widely studied, major factor affecting IAA production are pH, temperature and tryptophan concentration, nitrogen source, days of incubation, etc.8.
Results of optimization studies in Pseudomonas putida UB1 revealed that addition of L-tryptophan at concentration of 0.2 mg/mL at pH 7.5 and 96 hrs of incubation produced maximum IAA9. Rhizobium spp. produced the highest IAA (166 μg/mL) at a temperature of 36°C, pH of 6.5, an incubation time of 24hrs. and respectively tryptophan and NaCl concentrations of 1 g/L and 0.1 g/L10.
Wheat is one of the top three grains consumed worldwide. India is the world’s second largest producer of wheat, although rising production per capita is quite low in contrast to current demand11,12, use of chemical fertiliser is significantly enhances output, but its impact on ecosystem and soil health cannot be neglected13. Biofertilizers and compost application can reduce reliance on chemical fertilizers while also ensuring sustainable agriculture. According to reports, using compost, sludge, and Azotobacter has increased wheat production and nutritional content when compared to artificial fertiliser14. Microbial community composition of rhizosphere is unique to plant and is affected by various biotic and abiotic factors such as plant genotype, age of the plant and environmental factors, various anthropogenic activities etc., respectively.
Research on Arabidopsis thaliana has revealed that, while grown under identical conditions, the seven cultivars exhibited distinct rhizodeposit compositions as well as the development of a genotype-specific rhizobacterial community15. Moreover, the architecture of the root system is influenced by the rhizodeposit’s composition16. Because native plants are confined to a specific region, their rhizosphere microflora must be distinct and may have agricultural significance, though such studies are meagre. Pterocarpus marsupium Roxb (Family: Fabaceae) is a large, indigenous, deciduous tree from Northern Western Ghats. Because of their great medicinal value, all the parts of this species have been used in homoeopathic, ayurvedic, and unani medical systems. Its rhizosphere could provide promising PGPR that is significant to agriculture.
The current study aims to isolate and screen IAA-producing PGPR from rhizosphere of Pterocarpus marsupium Roxb’s. Optimization study for IAA production with selected isolates for parameters viz, pH, temperature, tryptophan concentration, and incubation period and their effect on root development of wheat plant was investigated.
Materials and Methods
Soil sample Rhizosphere soil of Pterocarpus marsupium Roxb (Location: Paud Ghat, Dist- Pune, Maharashtra, India) was collected from 20- 30 cm depth from the ground, soil firmly attached to the root was taken along with root hairs in a sterile container and was further processed in laboratory conditions.
Isolation and screening of bacteria from rhizospheric soil
10 gm of rhizosphere soil was inoculated in the sterile nutrient broth and incubated at 28oC for 48hrs. Serially diluted sample was plated on a sterile nutrient agar plate and incubated at 28oC for 3-4 days; colony characteristics of all obtained isolates were noted. All isolates were purified and preserved on nutrient agar slants and stored at 4oC for further use.
Quantitative screening of IAA producing bacteria
For quantitative screening of IAA production by bacterial isolates, Salkowsky reagent(0.5% FeCl3 in 70% perchloric acid)8 was used. The sterile Luria broth with pH 7 and 0.1mg/mL of tryptophan was inoculated with the test bacterial cultures, and incubated at 28oC for 7 days. The cultures were then centrifuged, 1ml of supernatant was added with 2ml of Salkwasky reagent and incubated at room temperature for 30 minutes in the dark reaction developed pink colour, intensity of colour was measured calorimetrically at 430 nm against blank. The standard curve of IAA, was prepared using the standard concentrations of IAA (Sigma Aldrich) in Luria broth, which was further used to calculate the concentration of IAA from test samples 6,17.
Detection of IAA by Thin layer chromatography (TLC)
The standard IAA (0.1 mg/mL in methanol) and crude IAA (produced in the previous experiment) were spotted on a 15 cm x 10 cm TLC plate (Silica gel Gf 254, thickness 0.25 mm) and separated in solvent system conatining n-butanol, ethyl acetate, ethanol, and water in the ratio of 3:5:1:118. The plate was then developed with Ehmen reagent( 98 mL of 35% HClO4 mixed with 2 mL of 0.5M FeCl3)15. The plate was heated at 90oC for 3-4 minutes for visualization of spots. Rf values of test samples and standard IAA were compared and those which coincide with standard IAA were identified16,17.
Optimization of culture condition for maximum IAA production for selected isolates
The effect of pH, incubation temperature, incubation period, and tryptophan concentration on IAA production was studied using classical method 6,8,10,18,19 for the five top IAA-producing isolates, the procedures for the same are listed below:
pH
To study effect of pH on IAA production, Luria broth at pH 5, 6, 7, and 8 was supplemented with 0.1 mg/mL of tryptophan, inoculated individually with bacterial isolates, and incubated for 4 days at 28 oC. Concentration of IAA in test samples were determined by using Salkowsky reagent.
Incubation temperature
Effect of temperature on IAA production was studied by inoculating test bacterial samples in Luria broth of pH 7 supplemented with 0.1mg/mL of tryptophan and incubated for 4 days at temperatures 25, 30, 35, 40oC with continuous shaking at 100 rpm. IAA from test samples was determined by using Salkowsky reagent.
Incubation period
Each test bacterial isolates was separately inoculated in Luria broth of pH 7 containing 0.1mg/mL tryptophan and incubated at 28oC in shaker incubator at 100 rpm. Sample from each flask was collected at 24hrs, 48hrs, 72hr, and 96hrs of incubation and cell free broth was assessed for IAA using Salkowsky reagent.
Tryptophan concentration
Effect of tryptophan concentration on IAA production was determined by inoculating Luria broth of pH 7 supplemented with L-tryptophan at concentration of 0.05, 0.1, 0.5, and 1mg/mL with test bacterial isolates separately and incubated for 4 days at 28oC, IAA from all tubes were determined by using Salkowsky reagent.
Effect of selected PGPR on growth of Wheat
Effect IAA producing PGPR was studied on growth and root architecture of Wheat (Triticum aestivum) MACS 6222 variety. MACS 6222 is high yielding, rust resistant and commonly grown variety in India especially Maharashtra. Wheat seeds were procured from MACS-Agharkar Research Institute (ARI), Hol farm, Dist-Pune Maharashtra India. Seeds were washed with few drops of Tween 20 and water thoroughly to remove detergent completely and further surface sterilized with 0.1% HgCl2 for 1 minute, and washed with sterile distilled water five times. Surface sterilized seeds were treated with each test bacterial inoculum containing 108cells/mL overnight, coated seed were dried and 20 seeds of each treatment were placed in a petri plate on moist paper towel. All the petri plates were incubated in at 25oC for 10 days. Watering was done as per requirement. The physiological parameters viz., germination time and germination percentage, plant height, fresh biomass, root morphology were noted. Experiment was carried out in triplicate.
Statistical analysis
The results of optimization of IAA are presented as means of three replicates ± standard deviation. Significance of PGPR treatment on plant growth and root development were statistically analysed using one-way analysis of variance (ANOVA) and means were compared using Dunnet multiple comparison test at p = 0.05 in Minitab18 statistical software .The graphs were plotted using MS excel and Minitab18 software.
Results and Discussion
A total of twenty four isolates were obtained from the rhizosphere of Pterocarpus marsupium Roxb., colony characteristics are mentioned in Table1. Isolates were further screened for quantitative IAA production using the Salkowsky reagent. All of the isolates displayed pink coloration, indicating that they are all capable of producing IAA (Fig1). Five bacterial isolates viz; Et1, Rp1, Rp5, Rp6, Rp9 with higher potential for IAA production (viz 50, 61,70, 76, 70 μg/mL respectively) were employed in further studies. ( Table2).
Table 1: Colony characteristics of bacterial isolates from rhizosphere of Pterocarpus marsupium Roxb.
|
Isolate |
Size( mm) |
shape |
Colour |
Margin |
Opacity |
Consistency |
elevation |
|
Et1 |
2 |
Circular |
Off white |
Entire |
Opaque |
Soft |
Flat |
|
Et2 |
2 |
Circular |
Cream |
Entire |
Opaque |
Soft |
Flat |
|
Et3 |
6 |
Circular |
Off white |
Irregular |
Opaque |
Soft |
Convex |
|
Et4 |
3 |
Circular |
White |
Entire |
Opaque |
Soft |
Umbonate |
|
Et5 |
4 |
Circular |
White |
Irregular |
Translucent |
Soft |
Umbonate |
|
Et6 |
5 |
Circular |
Creamy yellow |
Entire |
Translucent |
Sticky |
Flat |
|
Et7 |
3 |
Circular |
Cream |
Irregular |
Opaque |
Soft |
Convex |
|
Et8 |
2 |
Circular |
Light yellow |
Entire |
Opaque |
Soft |
Slightly raised |
|
Et9 |
1 |
Circular |
Cream |
Entire |
Translucent |
Soft |
Flat |
|
Et10 |
3 |
Circular |
Off white |
Undulate |
Opaque |
Soft |
Flat |
|
Et11 |
4 |
Circular |
Yellow |
Entire |
Opaque |
Soft |
Convex |
|
Et12 |
2 |
Circular |
Cream |
Entire |
Opaque |
Soft |
Erose |
|
RP1 |
4 |
Circular |
Cream |
Entire |
Translucent |
Soft |
Flat |
|
RP2 |
3 |
Circular |
Off white |
Entire |
Translucent |
Sticky |
Flat |
|
RP3 |
3 |
Circular |
Shiny white |
Entire |
Translucent |
Mucoid |
Flat |
|
RP4 |
4 |
Circular |
Cream |
Irregular |
Opaque |
Sticky |
Convex |
|
RP5 |
3 |
Circular |
White |
Entire |
Opaque |
Soft |
Crateriform |
|
RP6 |
1 |
Circular |
White |
Entire |
Opaque |
Soft |
Raised |
|
RP7 |
1 |
Circular |
Cream |
Entire |
Opaque |
Soft |
Flat |
|
RP8 |
3 |
Circular |
White |
Entire |
Opaque |
Soft |
Flat |
|
RP9 |
3 |
Circular |
Off White glossy |
Entire |
Opaque |
Mucoid |
Convex |
|
RP10 |
2 |
Circular |
Off white |
Entire |
Translucent |
Soft |
Flat |
|
Ed1 |
5 |
Circular |
Cream |
Entire |
Opaque |
Soft |
Raised |
|
PT1 |
3 |
Circular |
Off white |
Entire |
Opaque |
Hard |
Convex |
 |
Figure 1: IAA production by bacterial isolates obtained from rhizosphere of Pterocarpus marsupium Roxb, pink colour indicates presence of IAA. |
Table 2: Quantitative IAA production by bacterial isolates obtained from rhizosphere of Pterocarpus marsupium Roxb. using Salkowsky reagent.
|
Isolate |
(IAAμg/ml) |
Isolate |
IAA(μg/mL) |
|
Et1 |
50 |
Rp1 |
61 |
|
Et2 |
27 |
Rp2 |
26 |
|
Et3 |
24 |
Rp3 |
42 |
|
Et4 |
24 |
Rp4 |
37 |
|
Et5 |
28 |
Rp5 |
70 |
|
Et6 |
18 |
Rp6 |
76 |
|
Et7 |
24 |
Rp7 |
35 |
|
Et8 |
23 |
Rp8 |
32 |
|
Et9 |
25 |
Rp9 |
70 |
|
Et10 |
25 |
Rp10 |
29 |
|
Et11 |
16 |
Ed1 |
31 |
|
Et12 |
14 |
PT1 |
22 |
Optimization studies on IAA production
Bacteria produce IAA by tryptophan-dependent and tryptophan-independent pathways, however majority of bacteria synthesize high amount of IAA in presence of L- tryptophan, as tryptophan is a precursor of IAA18,20. The L-tryptophan content, temperature, incubation time, and medium pH were the factors that optimized for IAA production by top five producers using the classical method (One Factor at a Time). The current study’s findings show that tryptophan supplementation in media raises IAA production. Mohite( 2013)6 and Duca et al.( 2014)21 support tryptophan’s beneficial effects on IAA production. Maximum IAA was produced at 0.1mg/mL of tryptophan by all five isolates i.e 54, 62, 73, 78, 72 mg/mL, respectively. Tryptophan concentrations between 0.05 and 0.1 mg/ml produced almost equal amount of IAA, although, IAA production is reduced at higher tryptophan concentrations. These results validate the findings of Shokri & Emtiazi(2010)19, who found that higher tryptophan concentrations have a negative impact on IAA production. However, Bharucha et al.(2013)9 have also reported maximum IAA production by Pseudomonas putida UB1 at 0.2% tryptophan. (Fig 2a)
Metabolic activities of microorganisms are highly affected by physiological factors such as pH, temperature, macro, and micronutrient sources and their concentration, etc. Every organism has a cardinal range of the above factors for growth. IAA production is also affected by factors viz., temperature, pH, tryptophan concentration, nitrogen source, NaCl, incubation period etc.8,23,24.
There is a diverse report on the temperature requirement and incubation period for maximum IAA production. In the current investigation four isolates (Et1, Rp1, Rp5, Rp9) have produced maximum IAA i.e 50, 61, 71,71μg/mL respectively at 30oC and decreased further with increase in temperature; whereas RP6 produced maximum IAA (84.12 μg/mL ) at 35oC (Fig 2b). All the isolates produced highest IAA at 96 hrs. of incubation(Fig.2c). Results are in support of Patten&Glick(2002)25, they have obtained maximum IAA production by Pseudomonas putida. GR12–2 in 96 hrs and beyond 96hrs IAA concentration was found to be decreased; B. siamensis also produces maximum IAA at 35oC in 96 hrs26. Shokri and Emtiaz(2010)22 have reported 30oC and 72 hrs as an optimum temperature and incubation period for Paenibacillus, and Rhizobium strains. IAA production was found to be increased with the incubation period as IAA production occurs at the stationary phase of growth10. Rhodopseudomonas palustris produced maximum IAA (80.77± 2.13 μg/mL) at 35oC in 48hrs27.
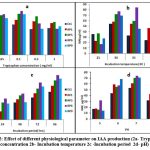 |
Figure 2: Effect of different physiological parameter on IAA production (2a- Tryptophan concentration 2b- Incubation temperature 2c -Incubation period 2d- pH). |
pH significantly affects the synthesis of IAA. A wide range of pH values have been found to be ideal for the synthesis of IAA27. In our investigation it was found that the synthesis of IAA is adversely affected by an acidic pH. At pH 7, all five isolates produced the highest amount of IAA, which ranges from 53 to 74 μg/mL. It was shown that Rp1 could produce almost the same amount of IAA (59.2 & 61 μg/mL) at pH values of 6 and 7, respectively; Rp6 produced the maximum amount of IAA (74 and 73 μg/mL, pH values of 7 and 8), and Rp9 produced nearly the same amount of IAA at pH values of 6 (68.71 μg/mL), 7 (71), and 8 (71.67g/mL) (Fig 2d). Mohite (2013)8 has also examined this fact, that distinct isolates generated maximum IAA at varying pH values. Lebrazi, Niehaus, et al. (2020)16 report that Rhizobium produces highest levels of IAA at pH 6.5, whereas Bharucha et al(2013)9 reported that Pseudomonas putida UB19 has a pH of 7.5.
Detection of IAA by thin-layer chromatography (TLC)
The developed chromatogram showed pink colour spots with all bacterial samples and standard IAA. The bacterial sample showed two spots each one with an Rf value of 0.91 which matches with standard IAA while another spot is of Rf value 0.23 which remains to be identified (Fig 3). Our results of TLC of IAA are consistent with Shokri & Emtiazi(2010)22 and Kang et al(2019)28 studies. On TLC plates, one more component that has not yet been named was found in addition to IAA.
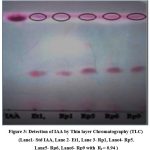 |
Figure 3: Detection of IAA by Thin layer Chromatography (TLC). |
(Lane1- Std IAA, Lane 2- Et1, Lane 3- Rp1, Lane4- Rp5. Lane5- Rp6, Lane6- Rp9 with Rf = 0.94 )
Effect of PGPR on Wheat growth and root system development
Beneficial effect of bacterial isolates Et1, Rp1, Rp5, Rp6 and Rp9 were evaluated on wheat growth. The statistical analysis of variance showed that all the five isolates significantly increase the plant height in comparison with untreated control as P = 0.00 at 0.05 % level of significance. Dunnets’s Simultaneous comparison at 95% confidence intervals also suggests means of height and biomass of all treatment are significantly different from untreated control mean (Fig 4a,4b,). Similarly analysis of variance of plant biomass give value of P =0.00 at 0.05% level of significance, Dunnet simultaneous comparison indicates that there is a significant difference between mean of untreated control and ET1, RP5 and RP6 but treatment RP1 and RP9 does not have significant difference in mean biomass with untreated control(Fig 4c and 4d).
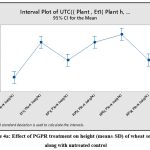 |
Figure 4a: Effect of PGPR treatment on height (mean± SD) of wheat seedlings along with untreated control. |
 |
Figure 4b: Comparison of height of wheat seedling with Individual treatment and untreated control by Dunnett simultaneous comparison at 95% CI |
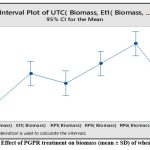 |
Figure 4c: Effect of PGPR treatment on biomass (mean ± SD) of wheat seedlings |
 |
Figure 4d: Comparison of biomass wheat seedling with individual treatment and untreated control by Dunnett simultaneous comparison at 95% CI |
Rp6 appears to be most promising among all five as it causes approximately 50% increase in plant height and biomass followed by Rp5 (40%) and Et1 (30%) (Table3,Fig5a), these results are in agreement with studies performed by Dahmani et al.(2020)29, in which plant growth promoting Bacillus megaterium strain (RmBm31) increases root biomass and positively develops root architecture of Arabidopsis thaliana. Similar to this, Ashrafuzzaman et al.(2009)30 showed that rice rhizosphere isolates generated IAA and impacted plant height and root length of rice seedlings.
Table 3: Effect of bioinoculation on height and biomass of wheat plant ( 10 days seedling)
|
Treatment |
Avg height(m) |
Biomass(g) |
|
Et1 |
0.16 ±0.01 |
0.14±0.018 |
|
Rp1 |
0.12±0.02 |
0.13±0.017 A |
|
Rp5 |
0.16±0.01 |
0.15±0.011 |
|
Rp6 |
0.17±0 |
0.17±0.009 |
|
Rp9 |
0.12±0.01 |
0.12±0.013 A |
|
UTC |
0.12±0.01 A |
0.11±0.009 A |
UTC- untreated control; Means are represented as average of three replicates ± S.D
Means not labelled with the superscript letter A are significantly different from the control level mean according to Dunnet Multiple Comparison Test at P = 0.05.
In our studies wheat seedlings treated with IAA-producing bacterial isolates Et1, Rp1, Rp5, Rp6, and Rp9 showed striking shift in the root system architecture, demonstrating significant expansion of lateral roots and root hairs in comparison to untreated control(Fig 5b).Several workers have previously described similar findings caused by inoculation with Bacillus altitudinis (strain FD48) in rice31, Phyllobacterium brassicacearum STM196 strain in Arabidopsis thaliana32. Auxins and cytokinins plays significant role in lateral root and root hair development32,33.
 |
Figure 5: a) Effect of bio inoculation treatment on wheat seedlings with UTC ( untreated control);b) Root morphology of PGPR treated and UTC (untreated control) on wheat seedlings |
According to Ambreetha et al.(2018)27 and López-Bucio et al.(2007)34, accumulation of IAA in the rice plant’s root system after treatment with Bacillus increases the number of roots and lateral roots, thickness, area, and volume of the roots, compared to untreated plant similar results were obtained by Patten & Glick(2002)25 in the treatment of mung bean seeds with Pseudomonas putida GR12-2.
Conclusion
The current investigation’s findings highlight the significance of the rhizosphere of the native plant Pterocarpus marsupium Roxb. as a source of IAA producing PGPR. Among five isolate Rp6 appears to be more promising as it produces high amount of IAA at diverse culture condition ( pH, temperature, incubation period etc.) hence it can enhance plant growth at diverse climatic conditions in the field. All the isolates under study enhanced wheat growth and development of lateral roots and root hairs. As a result, additional research is required to determine whether any of the chosen isolates have the potential to be a viable biofertilizer in the field settings. Therefore, it can be concluded that IAA producing isolates obtained from rhizosphere of Pterocarpus marsupium Roxb. species have a significant impact on plant growth and development and have prospective as a biofertilizer for sustainable agriculture.
Acknowledgement
The Authors are grateful to Dr. Sanjay Kharat, Principal, Modern College, Ganeshkhind, Pune and Dr. Vinay Kumar, Head, Department of Biotechnology and Dr. Rekha Gupta, former Head Department of Biotechnology, Principal B.G. College, Sangvi, Principal, T. C. College, Baramati for facilities and support.
Funding Sources
There are no funding sources.
Conflict of Interest
There is no conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Conflict of Interest
Authors declare that they have no conflict of interest.
Ethical Approval Statement
This article does not contain any studies with human participants or animals.
Author’s Contribution
Conceptualization and designing of the research work (VR, RB,SA ); Execution of lab experiments and data collection (VR ); Analysis of data and interpretation (VR ); Preparation of manuscript (VR,RB ).
References
- Haldar S, Sengupta S. Send Orders for Reprints to reprints@benthamscience.ae Plant-microbe Cross-talk in the Rhizosphere: Insight and Biotechnological Potential. 2015;(iii):1-7.
CrossRef - Hakim S, Naqqash T, Nawaz MS, et al. Rhizosphere Engineering With Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front Sustain Food Syst. 2021;5(February):1-23. doi:10.3389/fsufs.2021.617157
CrossRef - Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169(1):30-39. doi:10.1016/j.micres.2013.09.009
CrossRef - Ortiz-Castro R, Valencia-Cantero E, Lopez-Bucio J. The beneficial role of Rhizosphere microorganisms in plant health and productivity: Improving root development and nutrient acquisition. Acta Hortic. 2012;1009(October):241-250. doi:10.17660/actahortic.2013.1009.29
CrossRef - Raheem A, Shaposhnikov A, Belimov AA, Dodd IC, Ali B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch Agron Soil Sci. 2018;64(4):574-587. doi:10.1080/03650340.2017.1362105
CrossRef - Chandra S, Askari K, Kumari M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J Genet Eng Biotechnol. 2018;16(2):581-586. doi:10.1016/j.jgeb.2018.09.001
CrossRef - Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425-448. doi:10.1111/j.1574-6976.2007.00072.x
CrossRef - Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr. 2013;13(3):638-649. doi:10.4067/S0718-95162013005000051
CrossRef - Bharucha U, Patel K, Trivedi UB. Optimization of Indole Acetic Acid Production by Pseudomonas putida UB1 and its Effect as Plant Growth-Promoting Rhizobacteria on Mustard (Brassica nigra). Agric Res. 2013;2(3):215-221. doi:10.1007/s40003-013-0065-7
CrossRef - Lebrazi S, Fadil M, Chraibi M, Fikri-Benbrahim K. Screening and optimization of indole-3-acetic acid production by Rhizobium sp. strain using response surface methodology. J Genet Eng Biotechnol. 2020;18(1). doi:10.1186/s43141-020-00035-9
CrossRef - Sharma RC, Crossa J, Velu G, et al. Genetic gains for grain yield in CIMMYT spring bread wheat across international environments. Crop Sci. 2012;52(4):1522-1533. doi:10.2135/cropsci2011.12.0634
CrossRef - Crespo-Herrera LA, Crossa J, Huerta-Espino J, et al. Genetic yield gains in CIMMYT’S international elite spring wheat yield trials by modeling the genotype × environment interaction. Crop Sci. 2017;57(2):789-801. doi:10.2135/cropsci2016.06.0553
CrossRef - Pahalvi HN, Rafiya L, Rashid S, Nisar B, Kamili AN. Chemical Fertilizers and Their Impact on Soil Health BT – Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs. In: Dar GH, Bhat RA, Mehmood MA, Hakeem KR, eds. Cham: Springer International Publishing; 2021:1-20. doi:10.1007/978-3-030-61010-4_1
CrossRef - Mohamed MF, Thalooth AT, Elewa TA, Ahmed AG. Yield and nutrient status of wheat plants (Triticum aestivum) as affected by sludge, compost, and biofertilizers under newly reclaimed soil. Bull Natl Res Cent. 2019;43(1). doi:10.1186/s42269-019-0069-y
CrossRef - Ehmann A. The van URK-Salkowski reagent – a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr A. 1977;132(2):267-276. doi:10.1016/S0021-9673(00)89300-0
CrossRef - Lebrazi S, Niehaus K, Bednarz H, Fadil M, Chraibi M, Fikri-Benbrahim K. Screening and optimization of indole-3-acetic acid production and phosphate solubilization by rhizobacterial strains isolated from Acacia cyanophylla root nodules and their effects on its plant growth. J Genet Eng Biotechnol. 2020;18(1). doi:10.1186/s43141-020-00090-2
CrossRef - Rahman A, Sitepu IR, Tang SY, Hashidoko Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci Biotechnol Biochem. 2010;74(11):2202-2208. doi:10.1271/bbb.100360
CrossRef - Pollmann S, Düchting P, Weiler EW. Tryptophan-dependent indole-3-acetic acid biosynthesis by “IAA-synthase” proceeds via indole-3-acetamide. Phytochemistry. 2009;70(4):523-531. doi:10.1016/j.phytochem.2009.01.021
CrossRef - Myo EM, Ge B, Ma J, et al. Indole-3-acetic acid production by Streptomyces fradiae NKZ-259 and its formulation to enhance plant growth. BMC Microbiol. 2019;19(1):1-14. doi:10.1186/s12866-019-1528-1
CrossRef - Bunsangiam S, Thongpae N, Limtong S, Srisuk N. Large scale production of indole-3-acetic acid and evaluation of the inhibitory effect of indole-3-acetic acid on weed growth. Sci Rep. 2021;11(1):1-13. doi:10.1038/s41598-021-92305-w
CrossRef - Duca D, Lorv J, Patten CL, Rose D, Glick BR. Indole-3-acetic acid in plant-microbe interactions. Antonie van Leeuwenhoek, Int J Gen Mol Microbiol. 2014;106(1):85-125. doi:10.1007/s10482-013-0095-y
CrossRef - Shokri D, Emtiazi G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by taguchi design. Curr Microbiol. 2010;61(3):217-225. doi:10.1007/s00284-010-9600-y
CrossRef - Shen J, Yuan L, Zhang J, et al. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011;156(3):997-1005. doi:10.1104/pp.111.175232
CrossRef - Yuan CL, Mou CX, Wu WL, Guo Y Bin. Effect of different fertilization treatments on indole-3-acetic acid producing bacteria in soil. J Soils Sediments. 2011;11(2):322-329. doi:10.1007/s11368-010-0315-2
CrossRef - Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68(8):3795-3801. doi:10.1128/AEM.68.8.3795-3801.2002
CrossRef - Suliasih, Widawati S. Isolation of indole acetic acid (IAA) producing Bacillus siamensis from peat and optimization of the culture conditions for maximum IAA production. IOP Conf Ser Earth Environ Sci. 2020;572(1):0-11. doi:10.1088/1755-1315/572/1/012025
CrossRef - Pei LP. Production and optimization of indole-3-acetic acid by rhodopseudomonas palustris leong pei pei universiti teknologi malaysia. 2015.
- Kang SM, Shahzad R, Bilal S, et al. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019;19(1):1-14. doi:10.1186/s12866-019-1450-6
CrossRef - Dahmani MA, Desrut A, Moumen B, et al. Unearthing the Plant Growth-Promoting Traits of Bacillus megaterium RmBm31, an Endophytic Bacterium Isolated From Root Nodules of Retama monosperma. Front Plant Sci. 2020;11(February):1-15. doi:10.3389/fpls.2020.00124
CrossRef - Ashrafuzzaman M, Hossen FA, M. Razi Ismail, et al. Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth. African J Biotechnol. 2009;8(7):1247-1252.
- Ambreetha S, Chinnadurai C, Marimuthu P, Balachandar D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere. 2018;5:57-66. doi:10.1016/j.rhisph.2017.12.001
CrossRef - Contesto C, Milesi S, Mantelin S, et al. The auxin-signaling pathway is required for the lateral root response of arabidopsis to the rhizobacterium phyllobacterium brassicacearum. Planta. 2010;232(6):1455-1470. doi:10.1007/s00425-010-1264-0
CrossRef - Ortíz-Castro R, Valencia-Cantero E, López-Bucio J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav. 2008;3(4):263-265. doi:10.4161/psb.3.4.5204
CrossRef - López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, et al. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact. 2007;20(2):207-217. doi:10.1094/MPMI-20-2-0207
CrossRef

