Introduction
The purpose of bio-fertilization is to increase the population of beneficial microbes in the soil and to speed up microbial activities that boost the availability of nutrients that can be readily taken up by plants1. Although bio-fertilizers cannot replace conventional fertilizers, their usage can be controlled while supporting long term viable agricultural goals2. Microbial bio inoculants have been generated as an alternative for improving plant growth and eradication of disease but their wider implementation as formulatory product is yet to catch up, especially in developing nations.
Soil-borne bacteria, particularly the plant growth-promoting bacteria, invade the rhizosphere or plant roots aggressively and stimulate plant development3 lowering the demand for chemical fertilizers4. Bioinoculants in the form of Plant Growth Promoting Rhizobacteria (PGPR) take up a vital role in crop production and are economically useful to producers and consumers alike by safeguarding the soil under adverse conditions. Using PGPR as a bioinoculant results in long term production of agricultural yield. Bioinoculant formulations are produced by immobilization on a suitable carrier material, which could be an agricultural waste.
Waste proteins are eliminated hazardously causing pollution in the environment. Solid wastes are mostly generated by the agriculture sector. From production through consumption, agricultural solid wastes are generated at every link in the chain, depending on the type of agricultural product, how it is processed, and where it is expected to be used5. Organic fertilizers, which are the way out for sustainable agriculture, require the addition of high quality proteins. Cultivation of microbial biomass on organic solid substrate such as agrowastes through Solid State Fermentation (SSF) technology offers a viable option for bioformulation development6.
The present study explores the potentiality of agro wastes to act as solid substrate for mass production of indigenous rhizospheric bacteria and evaluates its potential to act as biofertilizer for plant growth promotion.
Objectives
Segregation of rhizospheric bacteria and sorting of traits of plant growth promotion.
Solid state fermentation of agrowaste by selected potential plant growth promoting strains and assessment of its population dynamics.
Evaluation of the efficacy of the prepared agrowaste based bioformulation in promoting growth of plant.
Materials and Methods
Isolation of Bacteria from the Rhizosphere
The root system of a month old healthy brinjal plant is separated from the plant body at the base of the stem and the larger soil particles are removed. 10 g of rhizosphere soil is weighed out and put to 100 mL of sterile water blank and shaken for 15 mins on a magnetic shaker. Serial dilutions were done and from the final dilutions, inoculation was done on the Nutrient agar plates. Incubation was done for 24-48 hrs at 35°C.
Screening of isolated bacterial strains for plant growth promoting characters and characterization of selected strain
The bacterial strains isolated were maintained in pure culture. The isolates were tested for plant growth promoting traits that is, nitrogen fixation, ammonia generation, phosphate solubilisation and siderophore synthesis.
Screening of Nitrogen-Fixing Activity
The twelve strains isolated were inoculated at the centre of glucose nitrogen free mineral medium (G-NFMM) agar plates containing bromothymol blue solution (BTB) Appearance of green colour indicated that the isolate had nitrogen fixing activity7. The coloured zone index was computed on the third day for the isolates that produced blue colored zones on the agar plate.

Screening for ammonia production
The generation of ammonia in each isolate was examined in accordance with Cappuccino and Sherman’s (1992)8. The ability of bacterial isolates to produce ammonia in peptone water was examined. Ten milliliters of peptone water were taken in tubes and fresh cultures of test organisms were allowed to grow for 48–72 hours at 28±2 °C. Each tube received 0.5 ml of Nessler’s reagent. A positive test result for ammonia production was the development of a brown to yellow colour.
Screening for siderophore production
Chrome azurol S agar medium plates were prepared and bacterial isolates were tested for the synthesis of siderophores9. 10 μl of 106 CFU/ml of test organism were spot inoculated into each plate, and the plates were then incubated at 28±2 °C for 48–72 hours. A yellow-orange halo that developed surrounding the growth was indicative of siderophore production.
Phosphate solubilization screening
Pikovskaya’s agar plates were used to screen each isolate for phosphate solubilization10. The test isolates were cultured for 4 days at 28±2 °C in 25 ml of Pikovskaya’s broth. The ratio of the overall diameter (colony and halo zone) to the colony diameter was used to compute the Solubilization Index (SI)11.
Screening for IAA production
A qualitative method was used to analyze the generation of IAA12 . Tryptophan (1mg/ml) was added to nutrient broth containing bacterial cultures and growth was allowed for 7 days at 35±2ºC. The cultures were centrifuged for 30 minutes at 3000 rpm. 4 mL Salkowski’s reagent (50 ml, 35% perchloric acid; 1 ml, 0.5M FeCl3) was combined with 2 mL of supernatant and 2 drops of orthophosphoric acid was added. Indole Acetic Acid (IAA) synthesis was suggested by the appearance of a pink color13. Absorbance at 530 nm was recorded by a Spectrophotometer. A standard curve made from pure IAA solutions (0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, and 65 μg ml-1) was used to calculate the concentration of IAA.
Selection of Bacterial isolates as bioagent for fermentation
Two of the bacterial strains showing plant growth promoting characteristics were selected for application as bioagent in the organic bioformulation.
Screening for compatibility between bacterial strains
Using the methodology of Fukui et al. (1994)14, the compatibility between the isolates of Pseudomonas and Bacillus was assessed. Both vertical and horizontal streaking of the bacterial strains were done next to one another on NA medium. After being incubated for 72 hours at room temperature (28±2°C), the inhibitory zone was noted on the plates. Compatibility or otherwise was ascertained by the absence or presence of inhibition zone respectively. Equal proportions of compatible strains were mixed in broth and vortexed for 30s to make a homogenous consortium.
Selection of agrowastes
Seven locally available and low-cost organic substrates was selected for the study. These were husk of rice (Rh), bran of rice and wheat (Rb and Wb respectively), decomposed cake of mustard oil (Dm), cow dung (Cd), vermicompost (V) and rice straw (Rs).
Solid state fermentation of agrowastes by selected bacterial strains
The chosen agricultural wastes were dehydrated, ground into particles with a mixer-grinder until they were about 40 mm in diameter, and then the powdered waste was sterilized in a hot air oven for two to three hours at 161°C. For the purpose of composting, 1×107 cfu/mL of bacterial culture was added to each gram of dry powdered material. As an adhesive, carboxymethyl cellulose was utilized. The initial 60–65% moisture content was kept constant. Up to 28 days, the composts were turned over every three to four days. Bacterial composting was allowed to occur in an incubator set at 30°C. In the cosubstrate, all substrates were present in equal amounts with both bacterial cultures, while the uninoculated substrate combination served as the control. Following a 28-day period, the biofertilizers were sealed in 250g polybags and kept in storage at 4°C.
Evaluation of population dynamics of the selected bacterial strains in the agrowaste based substrates
The viable population of the bacteria was determined at 15, 30, 45, 60, 75 and 90 days after storage (DAS) of the biofertilizer preparations. In case of CSPfBs, bacterial load consisted of the viable populations of both the bacteria. The technique of serial dilution plating of Waksman (1922)15 was complied with for deducing the number of colony forming units of bioagent at different days after storage. A thorough mixture of 1g of the respective formulations and 10 mL of autoclaved distilled water was prepared in a rotary shaker by shaking for 20 mins. Eight 9 mL sterile distilled water blanks were taken and serial dilution was performed by pipetting 1 mL from the stock solution to the first water blank and then followed sequentially by transferring 1mL from the preceding dilution to the subsequent ones. From each of the final dilutions from 10-5 to 10-8, 0.1 mL part was inoculated and spread on KMB plates for Pseudomonas fluorescens based formulation and NA plates for Bacillus subtilis based formulation and combined formulation, CSPfBf. Incubation was done at 28±1oC for 48 hrs and then the cfu/g formulations were enumerated. Five formulations with the relative maximum returns of cfu/g of bacterial load were selected and evaluated for plant growth promotion in brinjal plant.
Efficacy of the selected agrowaste based bioformulation in promoting plant growth
At 90 days after application of the agrowaste based bioformulations in soil, the biochemical parameters, yield and yield related traits of the treated plants were evaluated. To evaluate the efficacy of the agrowaste based bacterial biofertilizer products, the total carbohydrate content (Anthrone method), protein content (Lowry’s method) and chlorophyll estimation (Arnon method) of the treated plants were done.
Data were recorded on the following attributes of the brinjal plants processed with the bioformulations, to assess the effectiveness of the bioformulations.
Plant height (cm)
The length of the main stem from the base to the growing apex of the plant was measured at maturity. The height of each plant receiving a particular bioformulation was measured and the data averaged out.
No. of branches/plant
The total no. of primary branches/plant was recorded at maturity. The data of plants receiving a particular bioformulation was collected and the average calculated out.
No. of fruits/plant
The total no. of effective fruits from each plant in a replication was counted and averaged out.
Yield/plant (kg)
The total weight of fruit harvested at different dates from each plant/replication was calculated and averaged out to have yield/plant.
Average fruit weight (g)/plant
The average fruit weight was calculated from the total fruit weight per plant divided by the total no. of fruits/plant.
Mean leaf area (cm2)
The area of leaves on the primary branches of a plant for each replication was calculated and average found out and recorded.
The completely randomized block design was followed for the experiment conducted consecutively in the years 2021-22 and 2022-23 and data collected accordingly.
Soil Profile and Meteorological Parameters
The soil profile of the experimental site in the campus of Dakshin Kamrup College, Mirza, was done in the Soil Testing Laboratory of the Department of Agriculture, Govt. of Assam and is recorded below in Table 1
Table 1: Soil profile of the campus of Dakshin Kamrup College, Mirza, Kamrup, Assam
| PARAMETERS | OBSERVATION |
| pH | 4.80 (Acidic) |
| Organic Carbon (%) | 0.74 (Medium) |
| P2O5 (kg/ Acre) | 2.68 (low) |
| K2O (kg/ Acre) | 76.42 (Medium) |
| Texture | Sandy Clay Loam |
Result and Discussion
Isolation of bacteria from rhizosphere
Twelve strains of rhizosphere bacteria were preserved on NA slants at 4°C.
Selection and characterisation of isolated strains with plant growth promoting characteristics
Table 2 represents the properties of the isolated twelve bacterial strains with respect to plant growth promotion.
Out of the twelve rhizosphere strains isolated, R11 strain showed the highest diameter of coloured zone (20 mm) and nitrogen fixing ability along with the highest solubilisation index of 2.21. This is followed by R9 strain with diameter of coloured zone (18mm) and nitrogen fixing ability and a solubilisation index of 1.84. Both the R9 and R11 strains showed positive tests for ammonia, siderophore and IAA production.
Table 2: The performance of plant growth promoting traits given by the isolated bacterial strains
| Strains | N-fixing ability
(Diameter of coloured zone in mm) |
Ammonia production | Phosphate solubilisation
(Solubilization Index) |
Siderophore production | IAA production (µg/mL) |
| R1 | – | – | – | – | 22.43±0.79* |
| R2 | – | – | – | – | 25.75±0.91 |
| R3 | – | – | 1.44 | – | 26.78±0.69 |
| R4 | – | – | – | – | 28.25±0.82 |
| R5 | – | – | 1.32 | – | 29.25±0.97 |
| R6 | – | – | – | + | 38.43±0.82 |
| R7 | + (10) | + | 1.65 | + | 44.41±0.22 |
| R8 | + (13) | + | 1.50 | + | 41.75±0.93 |
| R9 | + (18) | + | 1.84 | + | 50.28±0.23 |
| R10 | – | – | – | + | 41.43±0.71 |
| R11 | + (20) | + | 2.21 | + | 51.28±0.41 |
| R12 | + (7) | + | – | + | 39.67±0.12 |
+ and – represent positive and negative; * Values are Mean±SE; The experiment was repeated twice with three replicates for each isolate
Cultural and Biochemical characterization of selected Bacterial strains:
The cultural characters of the two strains R9 and R11 on Nutrient Agar Media, King’s Medium B and Peptone Yeast Extract are shown in Table 3. The biochemical characterisation of the two strains is shown in Table 4. The cultural and biochemical characterisation of the R9 and R11 strains indicated these as Pseudomonas fluorescens (Pf) and Bacillus subtilis (Bs) respectively.
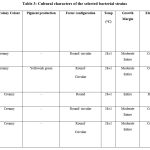 |
Table 3: Cultural characters of the selected bacterial strains |
Table 4: Biochemical characters of the selected bacterial strains
| Biochemical Test | Observation/ Result | |
| R9 | R11 | |
| Oxidase | + | + |
| Catalase | + | + |
| Nitrate reduction | + | + |
| KOH solubility | + | – |
| Tween 80 hydrolysis | – | – |
| Gelatin liquefaction | – | + |
| H2S production | – | + |
| Starch hydrolysis | – | + |
| Indole production | – | – |
| Methyl red | – | + |
| Voges Proskauer | – | – |
| Citrate utilization | + | + |
| Mode of glucose utilization | Aerobic | Aerobic |
| Acid Production from Glucose | + | + |
| Mode of sucrose utilization | Aerobic | Aerobic |
| Acid production from sucrose | + | + |
+ and – represent positive and negative
Population dynamics of the selected bacterial strains in the prepared agrowaste based substrates:
The bacterial load in the prepared agrowaste based substrates at 15, 30, 45, 60, 75 and 90 days after storage (DAS) are given in Table 5. The population load in all the agro waste based formulations showed an increasing trend from 15 DAS to 30 DAS and reached peak at 45 DAS. Thereafter there was a decreasing trend in the population load in all the agrowaste based formulations from 60 DAS to 75 DAS which further decreased at 90 DAS. This could be because the formulations include readily attainable carbon or other nutrients needed for the bacterial strains to proliferate and populate over time.
Table 5: Population load of the bacterial bioagents in the prepared formulations at different DAS
| Substrates | Population load at days after storage (DAS) (×108 cfu/g) | |||||
| 15 DAS | 30 DAS | 45 DAS | 60 DAS | 75 DAS | 90 DAS | |
| RhPf | 486.3
(10.68) |
581
(10.76) |
599.3
(10.77) |
347
(10.54) |
36.6
(9.56) |
29
(9.46) |
| WbPf | 688.3
(10.83) |
712
(10.85) |
787.6
(10.89) |
647.3
(10.81) |
86.3
(9.93) |
54
(9.73) |
| RbPf | 589.3
(10.77) |
677.6
(10.83) |
695.3
(10.84) |
521
(10.71) |
63.6
(9.80) |
42
(9.62) |
| DmPf | 752.6
(10.87) |
982
(10.99) |
1076.3
(11.03) |
688.3
(10.83) |
103
(10.01) |
63
(9.79) |
| CdPf | 568.3
(10.75) |
598.6
(10.77) |
619.6
(10.79) |
405.3
(10.60) |
47.6
(9.67) |
35.3
(9.54) |
| VPf | 886
(10.94) |
1170
(11.06) |
1293
(11.11) |
800.3
(10.90) |
277
(10.44) |
90
(9.95) |
| RsPf | 387.3
(10.58) |
476
(10.67) |
495
(10.69) |
287.6
(10.45) |
27
(9.43) |
20.6
(9.31) |
| RhBs | 507
(10.70) |
589
(10.77) |
606.3
(10.78) |
398.6
(10.60) |
41
(9.61) |
30
(9.47) |
| WbBs | 699.3
(10.84) |
865.6
(10.93) |
968
(10.98) |
661.6
(10.82) |
93
(9.96) |
61.6
(9.78) |
| RbBs | 634
(10.80) |
688.3
(10.83) |
702.6
(10.84) |
588.6
(10.76) |
77
(9.88) |
48.6
(9.68) |
| DmBs | 799
(10.90) |
1053.3
(11.02) |
1188
(11.07) |
746.6
(10.87) |
209.6
(10.32) |
80.3
(9.90) |
| CdBs | 564.6
(10.75) |
608.3
(10.78) |
627.6
(10.79) |
501.3
(10.70) |
59.6
(9.77) |
37.3
(9.57) |
| VBs | 894.6
(10.95) |
1202.3
(11.08) |
1388
(11.14) |
890.6
(10.94) |
300.6
(10.47) |
97.3
(9.98) |
| RsBs | 403.6
(10.60) |
488.6
(10.68) |
504.6
(10.70) |
305.3
(10.48) |
32
(9.50) |
24.3
(9.38) |
| CsPfBs | 939.6
(10.97) |
1308.6
(11.11) |
1508
(11.17) |
960.6
(10.98) |
310.6
(10.49) |
107.3
(10.03) |
| Effect of substrate carrier | S.Ed ± 4.37
CD0.05 7.42 |
S.Ed ± 3.35
CD0.05 5.69 |
S.Ed ± 3.08
CD0.05 5.23 |
S.Ed ± 1.97
CD0.05 3.34 |
S.Ed ± 1.78
CD0.05 3.02 |
S.Ed ± 1.50
CD0.05 2.55 |
Figs. within parenthesis indicate log transformed values
However, in the later phase of the assessment (90 days), the bacterial population most likely reduced markedly as a result of the nutrients being depleted from protracted use and the changed antagonistic microenvironment16,17. At more than 180 DAS, P. fluorescens strain-based talc or peat-based formulation could only hold out with a reduced population18. Among the formulations, CSBsPf, which consisted of a mixture of all the agowastes as well as both the bioagents, performed best giving the highest population load (11.17) at 45 DAS and a population load of 10.03 at 90 DAS. This is supported by the enhanced nutrient composition given by the mixture of agrowastes. Rice husk ash, vermiculite, peat, wheat bran, alginate and clay are reported to be good materials for use as a carrier19. As individual substrate for both Pf and Bs, vermicompost performed best with a population load of 9.95 of Pf and 9.98 of Bs at 90 DAS. This is sustained by the record that Vermicompost has higher nutrient value and better physico- chemical characters like pH, nitrogen, phosphorous, potassium, copper, manganese and iron that supports higher shelf life of Pseudomonas fluorescens and Bacillus sp20. Decomposed mustard oil cake, wheat bran, rice bran, cow dung and rice husk with a population load range of 9.79 to 9.46 for Pf and 9.90 to 9.47 for Bs at 90 DAS were the next best performers respectively. The largest stimulation of total bacteria and actinomycetes was observed with mustard cake (111.9 and 84.3%, respectively)21. Oil cakes greatly increased the proliferation of fungi, actinomycetes, and overall bacteria, leading to enhanced availability and holding of oxidizable C, N, and P. The farm yard manure (FYM) and wheat bran were recorded to be the most appropriate substrates for mass multiplication of Trichoderma spp22, among the variety of substances studied like peat soil, FYM, rice bran, wheat bran, and rice straw. Rice straw performed the least giving population load 10.69 of Pf and 10.70 of Bs at peak 45 DAS and 9.31 for Pf and 9.38 for Bs at 90 DAS. The lower performance of rice straw is supported by earlier reports that rice straw is a fresh agricultural material which can produce both beneficial and toxic products after degradation by microbial activities23.
Performance of selected agrowaste based bioformulation in plant growth promotion
The impact of the five agrowaste based bioformulations, chosen on the basis of shelf life, on the yield and yield related traits of the applied crop is given in Table 6.
The bioformulations CSPfBs, VBs and VPf when applied in soil, gave yield of 2.85 kg/ plant, 2.76 kg/ plant and 2.69 kg/ plant in brinjal plants 30 days after transplanting which were statistically significant at CD0.05 as compared to yield of DmPf and DmBs applied plants. Similar trends were also observed in growth parameters like plant height, no. of branches/ plant, leaf area, average fruit weight/ plant and no. of fruits/ plant. Earlier reports have also shown that application of vermicompost alongwith microbial inoculant showed increased plant growth and yield parameters24. Prior research demonstrated that inoculating the lettuce plant with strains of Pseudomonas and Bacillus, either independently or in combination, boosted plant growth characteristics relative to the control25, 26. It has been found that co-inoculation of the organic system with B. subtilis and P. fluorescens increases the biomass of both Gram-positive and Gram-negative bacteria27. This indicates that there may not be any competitive interactions between the native bacteria and the inoculants, probably as a result of the increased resource availability.
Table 6: Attainment of yield and yield related traits of crops applied with agrowaste based bioformulation
| Treatments | Leaf area (cm2) | Average fruit weight (g)/ plant | Yield/ plant (kg) | No: of fruits/plant | No: of branches/ plant | Plant height (cm) |
| DmBs | 164.6 | 162.96 | 2.30 | 14fgh | 15kl | 73.8 |
| DmPf | 156.6 | 156.96 | 1.88 | 13.8fgh | 14.2l | 72.6 |
| VPf | 174.0 | 169.56 | 2.69bc | 14.2fg | 16.4jk | 75.4 |
| VBs | 187.4 | 174.2a | 2.76dc | 14.8ef | 17.2ij | 79.4 |
| CSPfBs | 193.4 | 176.1a | 2.85db | 15.6e | 18.4i | 81.6 |
| Control | 100 | 138.28 | 1.11 | 12.4 | 11.2 | 69.6 |
| S.Ed.± | 2.47 | 1.83 | 0.135 | 0.588 | 0.889 | 1.726 |
| CD0.05 | 5.15 | 3.81 | 0.28 | 1.22 | 1.85 | 3.6 |
Values with similar superscript are not significantly different at 5% level of significance
Conclusion
The study indicates the potential of agrowastes to be used as effective substrate carriers for the formulation of bacterial biofertilizers. The cosubstrates of agrowastes with the inoculation of both Pseudomonas fluorescens and Bacillus subtilis strains performed best in promoting the yield and yield attributes. The agricultural wates, with high organic content has immense potential to be converted to value added products like biofertilizers through solid state fermentation. In the context of sustainable agriculture and importance of circular economy, the potential of agrowates inoculated with beneficial bacteria to be used as plant growth enhancers holds immense significance for further work in this direction.
Acknowledgement
The author is grateful to the Principal, Dakshin Kamrup College, Mirza, Kamrup, Assam, affiliated to Gauhati University for providing the infrastructure to complete the study.
Funding Sources
The present work has been conducted under the aegis of the office of the Principal, Dakshin Kamrup College, Mirza, under Gauhati University with no external funding source.
Conflict of Interest
There are no conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced throughout the research study
Ethics Approval Statement
This research did not involve human participants, animal, subjects, or any material that requires The ethical approval.
Author’s Contribution
( sole author): Dr. Gargi Chakravarty Assistant Professor Dakshin Kamrup College Mirza Kamrup Assam
Reference
- Mahdi S.S., Hassan G.I., Samoon S.A., Rather S.A, Showkat A.D. Biofertilizers in Organic Agriculture. Journal of Phytology 2010; 2(10): 42-54
- Okur N. A Review: Bio-Fertilizers- Power of Beneficial Microorganisms in Soils Biomed J Sci & Tech Res. 2018; 4(4) DOI: 26717/BJSTR.2018.04.0001076
CrossRef - Kaymak, H. C Plant growth and health promoting bacteria. Microbiology monographs, 2011; 18, 45-79.
CrossRef - Çakmakçi R., Figen Dönmez Figen, Aydın Adil, Şahin Fikrettin Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biology and Biochemistry 2006; 38 (6): 1482-1487 doi. https://doi.org/10.1016/j.soilbio.2005.09.019.
CrossRef - S. Braccoet al. Assessing the contribution of bioeconomy to the total economy: a review of national frameworks Sustainability 2018; 10(6), 1698; doi. https://doi.org/10.3390/su10061698
CrossRef - Vidhya D., Judia Harriet Sumanthy. Production of Biofertilizers from Agrowastes International Journal of Engineering and Techniques 2018; 4(1): 453-466
- Baldani J.I., Reis V.M., Videira S.S., Boddey L.H., Baldani V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil. 2014; 384: 413–431.
CrossRef - Cappuccino J. C. and Sherman N. Microbiology: A Laboratory Manual, 3. San Francisco. The Benjamin Cummings Publishing Co. Inc.; 1992
- Schwyn B and Neilands J.B. Universal chemical assay for the detection and determination of siderophores Anal Biochem. 1987; 60(1):47-56. doi: 10.1016/0003-2697(87)90612-9.
CrossRef - Gaur, A. C. Phosphate solubilizing micro-organisms as biofertilizer. Omega scientific publishers., New Delhi, 1990
- Edi–Premono, M.A. Moawad, and P.L.G. Vleck, Effect of phosphate solubilizing Pseudmonas putida on the growth of maize and its survival in the rhizosphere. Indonesian J. Crop Sci., 1996; 11: 13–23
- Bric, J. M., Bostock, R. M., and Silverstone, S. E. Rapid in situ assay for indoleacetic Acid production by bacteria immobilized on a nitrocellulose membrane. Applied and Environmental Microbiology, 1991; 57(2): 535–538. https://doi.org/10.1128/aem.57.2.535-538.1991
CrossRef - Loper, J. E., and Schroth, M. N. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology. 1986; 76: 386–389. doi: 10.1094/Phyto-76-386
CrossRef - Fukui, R., M.N. Schroth, M. Hendson and J.G. Hancock. Interaction between strains of pseudomonads in sugar beet spermospheres and their relationship to pericarp colonization by Pythium ultimum in soil. Phytopathology, 1994; 84: 1322-1330
CrossRef - Waksman S. A. A Method for Counting the Number of Fungi in the Soil. Journal of bacteriology, 1992; 7(3): 339–341.doi. https://doi.org/10.1128/jb.7.3.339-341.1922
CrossRef - Chakravarty G. and Kalita M.C Comparative evaluation of organic formulations of Pseudomonas fluorescens based biopesticides and their application in the management of bacterial wilt of brinjal (Solanum melongena L). African Journal of Biotechnology 2011; 10(37): 7174-7182
- Vidhyasekaran P and Muthamilan M Development of formulations of Pseudomonas fluorescens for control of chickpea wilt. Plant Dis. 1995; 79: 782-786.
CrossRef - Vidhyasekaran P, Rabindran R, Muthamilan M, Nayar K, Rajappan K, Subramanian N, Vasumathi K. Development of a powder formulation of Pseudomonas fluorescens for control of rice blast. Plant Pathol. 1997; 46: 291-297.
CrossRef - Jackson, A. M., Whipps, J. M., and Lynch, J. M. Production, delivery systems, and survival in soil of four fungi with disease biocontrol potential. Enzyme and microbial technology,1991; 13(8): 636-642.
CrossRef - Gandhi A. and Sivakumar K. Studies on shelf life of Azospirillum lipoferum, Bacillus megaterium and Pseudomonas fluorescens in vermicompost carrier. Journal of Phytology 2009; 1(2): 100–107
- Mondal, S., Das, R., and Das, A. C. A comparative study on the decomposition of edible and non-edible oil cakes in the Gangetic alluvial soil of West Bengal. Environmental monitoring and assessment,2014; 186(8): 5199–5207.
CrossRef - Sangeetha Panickar. and R. Jeyarajan. Mass multiplication of biocontrol agent Trichoderma spp. Indian J. Mycol. Pl. Pathol. 1993; 23: 328-330.
- Pornrapee Sarin and Nuntavun Riddech. Effects of Agricultural Residues as Carriers for Bio-fertilizer Production to Promote Tomato Growth in Saline Soil. Chiang Mai J. Sci. 2018; 45(4) : 1699-1712
- Manivannan, N. and Daniel Tomescu. Use of vermicompost as carrier material for microbial inoculants for enhanced crop production. Journal of Pure and Applied Microbiology. 2009; 3(1): 255-260.
- Cipriano MA, Lupatini M, Lopes-Santos L, et al. Lettuce and rhizosphere microbiome responses to growth promoting Pseudomonas species under field conditions. FEMS Microbiol Ecol. 2016; 92(12): fiw197. doi:10.1093/femsec/fiw197
CrossRef - Khosravi Anahita, Zarei Mehdi and RonaghiAbdolmajid. Effect of PGPR, Phosphate sources and vermicompost on growth and nutrients uptake by lettuce in a calcareous soil, Journal of Plant Nutrition. 2018; 41(1): 80-89, DOI: 10.1080/01904167.2017.1381727
CrossRef - Angelina E., Papatheodorou E. M., Demirtzoglou T., and Monokrousos N. Effects of Bacillus subtilis and Pseudomonas fluorescens Inoculation on Attributes of the Lettuce (Lactuca sativa L.) Soil Rhizosphere Microbial Community: The Role of the Management System. Agronomy, 2020; 10(9): 1428. MDPI AG
CrossRef

