Introduction
Heavy metal overabundance in agricultural soils and water due to sewage sludge discharge or air deposition raises the possibility of metals leaking into groundwater1. Metal levels in soil can vary widely due to both human activity and the geological origin of the soil. These concentrations can range from less than 1 mg/kg to 100,000 mg/kg. Natural aquatic and terrestrial ecosystems have been disrupted due to the outcome of excessive concentrations of various heavy metals in soil, including Cd+2, Cr+6, Cu+2, Ni+2, and Zn+2. While certain metals are static and determined, others are movable and have the potential to move via soil to groundwater or through plant roots (bioavailability)2. In contrast to Ni, Cu, and Zn, which are three crucial micronutrients for plant nutrition, cadmium has undefined positive consequences effects and may be hazardous to plants and animals if its concentrations surpass specified limits3.The physiology and biochemistry of the harmful impact of Cd in plants are probably comparable to those documented for additional heavy metals. The cleanup processes for heavy metal pollution are often expensive and environmentally harmful, making them less feasible for widespread implementation. These days, engineers and scientists have begun to develop inexpensive methods for cleaning contaminated regions that involve biomass, living plants, and microbes. One revolutionary method is the extraction of metals via microbial products. For instance, several microorganisms release Siderophores, which are molecules that bind metals with an excellent selectivity. Siderophores are low molecular weight complexing substances that bacteria and fungus produce when there is a lack of iron. They have a wide range of potential uses in bioremediation and iron chelation therapy5.There have been reports of siderophores, which attach to metals, particularly hazardous ones like lead and cadmium6.In addition, certain plant species have been found to be able to withstand higher than average concentrations of heavy metals or other harmful substances7. The seeds have been introduced to the pollutants in a few experiments. This study looked at capacity of groundnut to sprout and develop in the presence of Cd+2. The current research concentrates on building of siderophores by Streptomyces pactum OR958669, studying the relationship between plant development and siderophore production. Another study looked at the elimination of the negative impacts of Cd+2 through soil treatment with cadmium tolerant Streptomyces pactum OR958669. This research explores novel methods for addressing heavy metal pollution in agricultural soils, emphasizing the role of microorganisms and plants in mitigating contamination. By investigating siderophore production through Streptomyces pactum OR958669 and its impact on plant growth, the study offers insights into sustainable remediation strategies. The findings contribute to the development of cost-effective and eco-friendly solutions for improving soil quality and safeguarding ecosystem health.
Materials and Methods
Organisms
Streptomyces pactum OR958669 isolated from pesticide contaminated agricultural soil was used in the experiments. The soil was collected from Jamnagar, Gujarat, India.
Estimation of Siderophore producing ability of Actinomycetes
The potential of actinomycetes to build siderophores was assessed using the standard Chrome Azurol S (CAS) assay. Glassware was cleaned in deionized water after being washed with 3 mol/l hydrochloric acid (HCl) to eliminate iron before to the experiment8. The siderophore synthesis by isolated strain was estimated using both qualitative and quantitative techniques. The CAS reagent was produced in accordance with Schwyn and Neilands for both techniques. In brief, 121 mg of CAS was dissolved in 100 ml of distilled water, and this was followed by the addition of 20 ml of a 1 mM ferric chloride (FeCl3·6H2O) solution, which was prepared in 10 mM HCl. Under stirring, this solution was combined with 20 ml of a hexadecyl trimethyl ammonium bromide (HDTMA) solution. 729 milligrams of hexadecyltrimethylammonium bromide (HDTMA) were dissolved in 400 milliliters of distilled water to prepare an HDTMA solution. The CAS-HDTMA solution underwent sterilization prior to additional applications9.
Qualitative method for estimation of Siderophore producing ability
This experiment was carried out using a modified version of Hu and Xu’s technique10.By combining 100 ml of the CAS Chrome Azurol S reagent with 900 ml of sterilized LB Luria-Bertani (LB) agar medium, CAS agar petri plates were made. On each petri plate, isolated strain was spot-inoculated. A plate without inoculation served as the control. All petri plates were incubated at 30°C for 7 days post inoculation, and the development of an orange zone surrounding the colonies was observed11.
Quantitative method for estimation of Siderophore producing ability
By utilizing the supernatant from actinomycetes culture cultivated in Luria-Bertani (LB) broth medium, siderophore was quantitatively assessed. For this procedure, 1 milliliter of broth was transferred into a 1.5 milliliter centrifuge tube (Thomas Scientific, US) and subsequently sterilized. It was then inoculated with 10 milliliters of freshly grown actinomycetes culture (at a concentration of 108 colony forming units (CFU) per milliliter). For the isolated microbial strain, four replicate specimens (tubes) were assembled. Additionally, a control tube (containing un-inoculated broth) was also maintained. Isolated actinomycetes cultures were agitated at 10,000 rpm for 10 min after being incubated at 28°C for 48 hours; the supernatant was then used to calculate the siderophore. After mixing 0.5 milliliter of CAS reagent with 0.5 milliliter of supernatant from each culture for 20 minutes, optical density at 630 nm was measured (Spectrophotometer: Thermo Scientific, Evolution 201). The quantification of siderophore produced by the strains was conducted in terms of percent siderophore unit (psu), determined through the application of the following formula12.
Siderophore production(psu) = (Ar-As ×100)/Ar
Where, Ar = absorbance of reference (CAS solution and un inoculated broth), and As = absorbance of sample (CAS solution and cell-free supernatant of sample).
Preparation of inoculums for Pot Study involving Streptomyces pactum
Streptomyces pactum OR958669 was cultivated on starch nitrate agar, and the actinomycetes cells were harvested from a seven-day-old culture and suspended in sterile saline solution to achieve a concentration of 2×106 cells/ml.
Plant growth studies and analysis involving Streptomyces pactum
Pot culture experiments were conducted over a 10-week duration using 2-gram groundnut seeds. The seeds underwent surface sterilization in a 10% (w/v) sodium hypochlorite solution for 10 minutes, followed by five rinses with water. For three days, the sterile seeds sprouted on damp sterile cotton. Four sets of pots were arranged: The first one remained without any inoculation (control), the second was inoculated with Streptomyces pactum OR958669, the third was heavy metal (Cadmium, 1000ppm) with Streptomyces pactum OR958669 and fourth was only heavy metal (Cadmium). For control, only water was added. This concentration was chosen because the strong strain in the study can withstand heavy metal cadmium concentrations of up to 1000ppm. The nutrient solution had the following composition, in mM: KH2PO4, 1.0; KNO3, 5; Ca (NO3)2, 5; MgSO4, 2; Fe- EDTA, 0.1; H3BO3, 0.005; MnCl2, 0.010; ZnSO4, 0.008; CuSO4,0.004; (NH4) Mo7O24, 0.0002. Following an initial growth phase of 15 days, the healthiest seedlings were chosen for further analysis and Cd was added as Cd (Cl2) (CdCl2 is a salt where Cd ion has 2+ valency and Chloride ion has -1 valency, when added to the water based nutrient solution Cd2+ dissociated from the salt) to the nutrient solution and Each pot was irrigated twice a week with only 200 milliliters of water, and additional irrigation with distilled water was provided to the plants as necessary. Each pot was rinsed with 200 milliliters of sterile distilled water per week. Following a period of 10 weeks, the plants were harvested and subjected to analysis. The root depth, shoot length, and number of leaves were measured and recorded. The shoot and root systems were separated, then subjected to oven-drying at 65°C for 10 days. Dry weights were subsequently determined for each sample.
Proline contents
Proline levels in the untreated and treated plants were measured. (The first one remained without any inoculation (control), the second was inoculated with Streptomyces pactum OR958669, the third was heavy metal (Cadmium) with Streptomyces pactum OR958669 and fourth was only heavy metal (Cadmium)). The method given by Bates and his team for estimating acid ninhydrin was used to estimate this. 10 ml of 3% aqueous sulfosalicylic acid were combined with two milliliters of water extract. In a test tube, 2 milliliter of this mixed solution was combined with 2 ml of acid ninhydrin-reagent, 2 ml of glacial acetic acid, and the reaction was allowed to run for an hour at 100°C. The reaction was halted by cooling the mixture in an ice bath. Using 4 ml of toluene as an extraction solvent, the reaction mixture was rapidly stirred for 15 to 20 seconds. The toluene containing the chromatophore was aspirated from the aqueous phase, brought to room temperature, and utilized as a blank to measure the absorbance at 520 nm. The proline concentration was ascertained using a standard curve in accordance with Payne’s 1993 methodology13.
Soluble Sugars Contents
The another one sulphoric acid technique, first proposed by Fales in 1951 and later developed by Schlegel and Badour, was used to quantify the number of soluble sugars14.
Phosphorus, N, K and Mg Concentration were calculated following acid digestion using a Shimadzu Atomic Absorption Flame Spectrophotometer (Model AA-640-12) in accordance with the procedures outlined by Allen15.
Total Chlorophyll Content
The amount of total chlorophyll, as well as the amounts of chlorophyll a and chlorophyll b, were measured using Arnon (1949). One-gram leaves of groundnut was obtained and blended with cold 80% acetone (v/v) in a clean mortar pestle to be carefully mashed and gently chopped. Adjust the volume to 25 ml with 80% acetone for the centrifugation, then centrifuge for 15 minutes at 3000 rpm. A fresh tube was used to transfer the clear suspension, and measurements of the optical density (OD) were taken at 645 and 663 nm. 80 percent acetone (v/v) was used as the blank. The following equation was utilized to ascertain the quantities of chlorophyll a and chlorophyll b16.
Where ,

Results and Discussions
The advancement in breeding superior crop varieties has led to the increased reliance on synthetic fertilizers. The health of the soil has declined as a result. The great utilization and control of soil vitality and physical qualities, which depend on soil biological processes and ecological diversity, have been developed in an effort to boost crop yield. An effective alternative to promote plant development is plant microbe contact. It was seen that an orange-colored zone formed surrounding the isolated colonies, indicating that our potent strain Streptomyces pactum OR958669 was producing siderophores. The generation of siderophore was found to be positive in potent strain used in the investigation. On CAS agar, Streptomyces pactum OR958669 produced siderophores at their highest level.
Based on the extent of the halo that formed on CAS agar, the qualitative siderophore production was roughly approximated. For measuring the quantity of siderophore production, liquid culture medium and CAS reagent are used.
The utilization of siderophores by bacteria to attach and hold metals besides iron serves as a crucial opposition strategy against metal toxicity. This mechanism is vital because hazardous metals can permeate bacterial cells, leading to detrimental effects on cellular functions and viability. By producing siderophores capable of binding these non-iron metals, bacteria can effectively reduce their availability in the surrounding environment. This action prevents the toxic metals from entering the bacterial cell and causing damage to essential biomolecules and cellular structures. Strategy utilized by bacteria to reduce metal availability since hazardous metals may penetrate the bacterial cell17. Siderophores play a key role in bacterial metal resistance. Studies have shown that bacteria producing siderophores, such as Pseudomonas aeruginosa strains, exhibit enhanced resistance to various metals compared to strains that do not produce these molecules18. This observation underscores the importance of siderophores as a defense mechanism against metal toxicity in bacterial populations. In order to prevent metal toxicity, S. acidiscabies and S. tendae siderophores able to bind nickel or cadmium, respectively19,20. According to these results, cells react to dangerous metal concentrations by producing siderophores, which chelate the free heavy metals via generating a metal-siderophore complex that cannot enter the cell.
Table 1: Highest spore productivity by Streptomyces pactum
| Sr. No. | Potent strains | Absorbance (630nm) | Qualitative analysis | Quantitative analysis (psu) |
| 1. | Control | 1.09 | – | – |
| 2. | Streptomyces pactum | 0.611 | +++ | 43.94 |
+++ (High production), – (No production)
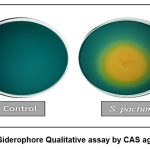 |
Figure 1: Siderophore Qualitative assay by CAS agar method |
 |
Figure 2: Siderophore Quantitative assay using liquid culture media |
In the present research, Fig.1 and Fig.2 showed qualitative and quantitative study of Streptomyces pactum OR958669 respectively. The strain was exhibited an orange halo after 7 days of incubation at 30°C on CAS agar plate and produced 43.94% siderophore. Thus, Streptomyces pactum OR958669 was considered positive for siderophore production.
Ground nut seeds were steeped in Streptomyces pactum culture filtrate that had been made with Cd at a concentration of 1000 ppm. Ground nut grains were planted on sandy soil for 10 weeks with Cd irrigation. Plants were treated with,
Control (without any inoculation)
Inoculation with Streptomyces pactum OR958669
Inoculation with heavy metal (Cadmium) and Streptomyces pactum OR958669
Inoculation with only heavy metal (Cadmium)
While the phosphorous and nitrogen substance of the plants were not significantly affected by Cd concentration, The concentrations of potassium and magnesium within the shoots exhibited a marked decline in direct proportion to the escalating levels of cadmium exposure (Table 3). Inoculating the soil with Streptomyces pactum could contribute to reducing the impacts of Cd on the K, Mg, N, and P contents of the investigated plant at 1000 ppm of Cd.
The Cd metal has a deleterious impact on the amount of chlorophyll (a+b) in leaves. Heavy metal or Streptomyces inoculation considerably enhanced the amount of soluble carbohydrates and proline in the shoot system compared to the control. (Table 3).
Belimov and his colleagues discovered eleven bacterial strains that could produce indole acetic acid and/or siderophores from mining scraps that was heavily polluted with Cd, sewage sludge, and Cd-enriched soils in the rhizosphere of Indian mustard (Brassica juncea L. Czern.) seedlings21. He also claims that previously cadmium-tolerant microbial strains have high tolerance to other metals like Zn, Cu, Ni, and Co. Two of the detected bacteria, Azotobacter vinelandii MM1 and Streptomyces spp. MM1017, generated IAA with favorable results, and soaking wheat seeds in both culture filtrates significantly more germination22. The possible impact of Cd on ground nut development in the presence and absence of Streptomyces was studied since it is thought to be a significant, potent environmental pollutant. Almost all of the observed characteristics were decreased by the rising concentrations of Cd as a heavy metal (HM). Since roots came into direct interaction with Cd in the supplying solution, they had greater Cd levels than shoots and were therefore more susceptible to the detrimental impacts of that heavy metal. At the maximum Cd levels, adverse impacts of Cd on plant development were found. In order to protect above-ground plant organs from exposure to heavy metals, Mazhoudi and his team theorized that roots play a protective role23. Similar research was conducted by Hussein and his colleagues on the effects of the heavy metal Lead Nitrate (Pb (NO3)2) on the length, fresh and dry weights, total protein amount and lead absorption by the roots and shoots of Zea mays. They asserted that all of the measures decreased due to the greater lead concentrations24.
Metal poisoning caused the growth retardation by damaging different physiological and metabolic systems. On the other hand, Atriplexhalimus, a potential halophyte plant for the rehabilitation of heavy metal contaminated environments, despite the fact that Cadmium (Cd) and lead (Pb) frequently induce a reduction in chlorophyll content and biomass, impacting water relations within this plant species.
Even tolerant or accumulator species can exhibit visual indications including growth and biomass decreases as well as structural changes in their roots when exposed to harmful heavy metals25. The size and quantity of xylem vessels, water chlorophyll a synthesizing potential, and plant hormonal balances were all decreased by cadmium26,27.
Increased heavy metal concentrations are hazardous to productive growth of Pisum sativum, and chlorophyll and proline content also demonstrated a progressive shift28. Different levels of Cd tolerance have been found in several plant species, and these differences have been linked to genetic or physiological characteristics, like the existence of inhibitors in roots that caused Cd to be allocated to apo plastic areas29. To be able to decrease oxidative damage and raise their Cd tolerance, plants can boost the activities of antioxidant enzymes30.
The creation of plant inoculant systems beneficial for phytoremediation of contaminated soils and the improvement of growth of the metal-accumulating plant Brassica juncea in the existence of harmful Cd levels are both promising applications for some heavy metal resistant bacteria. According to our findings, adding Streptomyces pactum OR958669 to soil improved plant development even when there were heavy metals present, and it also prevented plants from accumulating Cd. Similar to this, Streptomyces was identified and improved plant development in salty environments.
 |
Figure 3: (a) Control (without any inoculation), (b) Inoculation with Streptomyces pactum, (c) Inoculation with heavy metal (Cadmium) and Streptomyces pactum and (d) Inoculation with only heavy metal (Cadmium). |
 |
Figure 4: (a) Control (without any inoculation), (b) Inoculation with Streptomyces pactum, (c) Inoculation with heavy metal (Cadmium) and Streptomyces pactum and (d) Inoculation with only heavy metal (Cadmium). |
Table 2: Effect of Cd (1000ppm) on root depth (cm), shoot length (cm) and root and shoot dry weight (g/plant) of Ground nut plants cultivated in sterile soil with different treatments.
| Treatments | Root | Shoot | |||||
| Depth (cm) | Fresh
weight (g/plant) |
Dry
weight (g/plant) |
Length (cm) | Fresh weight
(g/plant) |
Dry weight (g/plant) | Number of leaves | |
| Control | 11.4 | 4.4 | 2.3 | 33.1 | 6.6 | 1.3 | 18 |
| S. pactum | 13.9 | 5.2 | 3.3 | 36.6 | 8.9 | 2.3 | 18 |
| Cd + S. pactum | 9.57 | 2.3 | 1.7 | 24.4 | 4.9 | 1.0 | 14 |
| Cd | 6.6 | 1.1 | 0.9 | 16.3 | 3.2 | 0.6 | 10 |
Cd: Plants treated with Cadmium, Cd+ S. pactum: Plants treated with Cadmium in soil previously inoculated with S. pactum
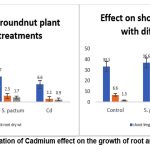 |
Figure 5: Graphical representation of Cadmium effect on the growth of root and shoot of Ground nut plant |
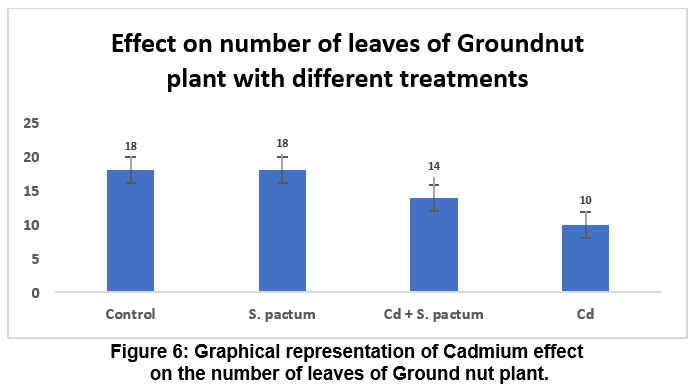 |
Figure 6: Graphical representation of Cadmium effect on the number of leaves of Ground nut plant |
Following the incubation period, observations were made regarding the root and shoot development as well as leaf count of germinated seedlings. (Fig 5 and Fig 6).
Root Growth
Depth: The treatment with S. pactum alone shows the greatest root depth, suggesting that it promotes root growth. In contrast, cadmium exposure (Cd) significantly reduces root depth, indicating toxic effects on root development. Interestingly, the combined Cd + S. pactum treatment shows root depth reduction but not as severe as Cd alone, suggesting some mitigation by S. pactum.
Fresh Weight: Follows a similar pattern to root depth, with S. pactum promoting growth and Cd hindering it. The mitigating effect of S. pactum in the presence of Cd is also observed here but to a lesser extent.
Dry Weight: The increase in dry weight under S. pactum treatment suggests increased biomass accumulation, which is beneficial for plant health. Cadmium exposure drastically reduces dry weight, showing the toxic effect on plant biomass. The combined treatment shows some mitigation, as seen in fresh weight
Shoot Growth
Length: Shoot length follows a similar trend, with S. pactum promoting longer shoots, while Cd significantly stunts shoot growth. The combination treatment shows a lesser degree of stunting, suggesting some protective effect of S. pactum against Cd toxicity.
Fresh Weight: The patterns here mirror those of shoot length, with S. pactum enhancing growth and Cd reducing it. The combination treatment suggests a partial mitigation of Cd’s negative effects.
Dry Weight: Similar to other metrics, dry weight is highest with S. pactum treatment and lowest with Cd, with the combined treatment showing intermediate values.
Number of Leaves: The number of leaves also follows this trend, with the highest number in both the control and S. pactum treatments, a significant reduction under Cd stress, and a lesser reduction when S. pactum is applied alongside Cd.
Table 3: Effect of Cd (1000ppm) on shoot content of minerals, chlorophyll content, sugars and proline of Ground nut plants grown in sterile soil with different treatments.
| Treatments | Shoot content of metal ions
(mg/g) |
Shoot analysis (mg/g) | |||||
| K+ | Mg++ | N + | P++
|
Chlorophyll (a+b) | Proline | Soluble sugar | |
| Control | 17.4 | 4.6 | 18.2 | 14.0 | 9.18 | 0.39 | 63.0 |
| S. pactum | 14.9 | 5.8 | 15.62 | 10.2 | 9.90 | 0.39 | 78.0 |
| Cd + S. pactum | 11.9 | 5.11 | 16.4 | 10.8 | 3.11 | 0.80 | 120.0 |
| Cd | 11.8 | 6.6 | 20.6 | 10.4 | 2.21 | 0.70 | 80.9 |
Cd: Plants treated with Cadmium, Cd+ S. pactum: Plants treated with Cadmium in soil previously inoculated with S. pactum
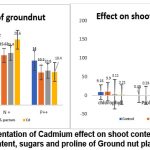 |
Figure 7: Graphical representation of Cadmium effect on shoot content of minerals, chlorophyll content, sugars and proline of Ground nut plants |
Fig 7 indicates the physiological parameters and metal ions present in shoot in Ground nut plant.
Metal Ions in Shoots (mg/g)
K+ (Potassium)
There’s a decrease in potassium content under both Cd and Cd + S. pactum treatments compared to the control, suggesting cadmium stress reduces potassium uptake or retention.
Mg++ (Magnesium)
Magnesium levels are somewhat variable; interestingly, the plants under Cd stress alone show the highest magnesium content, which could indicate a compensatory uptake or altered metabolism under heavy metal stress.
N+ (Nitrogen)
Nitrogen content is highest under Cd stress alone and lowest with S. pactum treatment, indicating that cadmium may affect nitrogen assimilation or that S. pactum may influence nitrogen uptake or utilization differently.
P++ (Phosphorus)
Phosphorus content decreases under all treatments compared to control, with the least decrease in the Cd + S. pactum treatment, suggesting that S. pactum might mitigate the impact of cadmium on phosphorus uptake to some extent.
Physiological Parameters
Chlorophyll (a+b)
Chlorophyll content is significantly reduced under cadmium stress, with or without S. pactum, indicating that cadmium severely affects photosynthesis. However, S. pactum alone slightly increases chlorophyll content compared to control, suggesting a positive effect on photosynthetic machinery or chlorophyll synthesis.
Proline
Proline levels increase under cadmium stress, which is a common response to metal stress and indicative of an adaptive mechanism to counteract oxidative damage. The presence of S. pactum further enhances proline accumulation, which might suggest a synergistic effect in stress response.
Soluble Sugar
Soluble sugar content significantly increases under cadmium stress, especially with the addition of S. pactum, indicating an alteration in carbohydrate metabolism possibly as a stress response or a protective mechanism against osmotic stress.
Conclusion
Heavy metal toxicity affects practically all plants; however, the severity of the toxicity varies from plant to plant. Consuming polluted plants as food or feed may have a severe effect on human health. Nevertheless, due to the high abundance of Streptomyces pactum OR958669 and their capacity to generate siderophore as secondary compounds, our hypothesis posits that Actinobacteria could serve as a promising reservoir of cadmium-binding compounds. These compounds, when synthesized in excess or in conjunction with plants, could be harnessed for soil or water decontamination purposes, along with other prospective biotechnological applications. In conclusion, our findings showed that ground nut has a significant capacity to develop under Cd stress and that inoculation with Cd resistance increases plant growth and Cd tolerance of this species. These findings possibly strongly connected to siderophore production, morphological and physiological characteristics.
Acknowledgement
The authors extend their gratitude to the Atmiya University for their support, enabling the researchers to actively participate in crop science-related research endeavors.
Funding Sources
The authors express their sincere appreciation to the Government of Gujarat for the financial assistance provided under its ‘SHODH SCHEME’ with Reference No. 202101241.
Conflict of Interest
There is no conflict of interest for the writers.
Data Availability Statement
All data pertinent to the current study are presented within the manuscript.
Ethics Approval Statement
Not applicable to this research.
Author’s Contribution
Ms. Tasnim Musani conducted the experiment and analyzed the data, while Dr. Mousumi Das supervised the experiment and contributed insights.
References
- Kabata-Pendias, A. and H. Pendias. Trace element in soils and plants, 1st ed. Boca Raton, F1: CRC Press. 1992;365.
- Gardea-Torresdey JL, Polette L, Arteaga S, Tiemann KJ, Bibb J, Gonzalez JH. Determination of the content of hazardous heavy metals on Larrea tridentata grown around a contaminated area. In: Proceedings of the Eleventh Conf. on Hazardous Waste Research,(HSRC/WERC Joint Conference on the Environment), Edited by LR Erickson, DL Tillison, SC Grant, and JP McDonald, Albuquerque, NM. ; 1996:660-669.
- Adriano, D. C. Trace Elements in the Terrestrial Environment. 533 Seiten, 99 Abb., zahlr. Tab. Springer-Verlag, New York, Berlin, Heidelberg, Tokyo. Preis. 1986;228.
- Meagher RB. Phytoremediation of toxic elemental and organic pollutants. CurrOpin Plant Biol. 2000;3(2):153-162.
CrossRef - Raymond KN, Müller G, Matzanke BF. Complexation of iron by siderophores a review of their solution and structural chemistry and biological function. Struct Chem. Published online 1984:49-102.
CrossRef - Yarnell A. Nature’s tiniest geoengineers. Chem \& Eng news. 2003;81(42):24.
CrossRef - Raskin I, Ensley BD, others. Phytoremediation of Toxic Metals. John Wiley and Sons; 2000.
- Shirling EBT, Gottlieb D, others. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966;16(3):313-340.
CrossRef - Cabaj A, Kosakowska A. Iron-dependent growth of and siderophore production by two heterotrophic bacteria isolated from brackish water of the southern Baltic Sea. Microbiol Res. 2009;164(5):570-577.
CrossRef - Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160(1):47-56.
CrossRef - Qing-Ping H, Jian-Guo X. A simple double-layered chrome azurol S agar (SD-CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. African J Microbiol Res. 2011;5(25):4321-4327.
CrossRef - Louden BC, Haarmann D, Lynne AM. Use of blue agar CAS assay for siderophore detection. J Microbiol \& Biol Educ. 2011;12(1):51-53.
CrossRef - Payne, S. M. Iron acquisition in microbial pathogenesis. Trends in microbiology. 1993;1(2):66-69.
CrossRef - Bates LS, Waldren RP a, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205-207.
CrossRef - Schlegel H-G. Die verwertungorganischersäurendurch Chlorella imlicht. Planta. 1956;47:510-526.
CrossRef - Raj S. N., ShettyN. P., Shetty H. S. Seed Bio-Priming with Pseudomonas Fluorescens Isolates Enhances Growth of Pearl Millet Plants and Induces Resistance against Downy Mildew. International Journal of Pest Management, 2004; 50(1): 41-48.
CrossRef - Allen, S. E.; Grimshaw, H. M.; Parkinson, J.A.; Quarmby, C.; Roerts, J. D. 1974. Chemical Materials. of Blackwell Ecological Scientific Publications, Oxford, London. 1974;565.
- Schalk IJ, Hannauer M, Braud A. New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol. 2011;13(11):2844-2854.
CrossRef - Braud A, Geoffroy V, Hoegy F, Mislin GLA, Schalk IJ. Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Environ Microbiol Rep. 2010;2(3):419-425.
CrossRef - Dimkpa C, Svatoš A, Merten D, Büchel G, Kothe E. Hydroxamate siderophores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can J Microbiol. 2008;54(3):163-172.
CrossRef - Dimkpa CO, Merten D, Svatoš A, Büchel G, Kothe E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J Appl Microbiol. 2009;107(5):1687-1696.
CrossRef - Belimov AA, Hontzeas N, Safronova VI, et al. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem. 2005;37(2):241-250.
CrossRef - Aly, M.M, El Sayed, H. E. A., Jastaniah, S. Synergistic Effect between Azotobactervinelandii and Streptomyces sp. isolated from saline soil on seed germination and growth of wheat plant. J Am Sci. 2012;8(5):667-676.
- Mazhoudi S, Chaoui A, Ghorbal MH, El Ferjani E. Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum, Mill.). Plant Sci. 1997;127(2):129-137.
CrossRef - Hussein, A. , Abbas, N. , Arshad, F. , Akram, M. , Khan, Z. , Ahmad, K. , Mansha, M. and Mirzaei, F. Effects of diverse doses of Lead (Pb) on different growth attributes of Zea-Mays L. Agricultural Sciences. 2013;4:262-265.
CrossRef - Gomes MP, Martins GA, Carneiro MMLC, Soares ÂM, others. Cd-tolerance markers of Pfaffia glomerata (Spreng.) Pedersen plants: anatomical and physiological features. Brazilian J Plant Physiol. 2012;24:293-304.
CrossRef - Wójcik M, Vangronsveld J, D’Haen J, Tukiendorf A. Cadmium tolerance in Thlaspicaerulescens: II. Localization of cadmium in Thlaspicaerulescens. Environ Exp Bot. 2005;53(2):163-171.
CrossRef - Skrebsky EC, Tabaldi LA, Pereira LB, et al. Effect of cadmium on growth, micronutrient concentration, and $δ$-aminolevulinic acid dehydratase and acid phosphatase activities in plants of Pfaffia glomerata. Brazilian J Plant Physiol. 2008;20:285-294.
CrossRef - Borah M, Devi A. Effect of heavy metals on Pisum sativum Linn. Published online 2012.
- Marchiol L, Leita L, Martin M, Peressotti A, Zerbi G. Physiological Responses of Two Soybean Cultivars to Cadmium.; 1996.
CrossRef

