Introduction
Paddy fields are a vital part of the environment and ecological systems of Kerala. More than providing food in the form of rice and non-rice foods such as fish and prawns, rice paddies provide other ecosystem services such as regulation of quantity and quality of water, ground water recharging, flood control, decomposition of organic wastes, soil formation, biological nitrogen fixation, soil erosion and landslide prevention, and biodiversity conservation1,2,3,4,5 . Rice ecosystems of Kerala are unique as these are greatly influenced by the regional climate and the land forms.
Rice is one of the major cultivated crops of Kerala, but its cultivating area is found to be decreasing and the tendency to keep paddy fields fallow is probably increasing every year. For the past few decades, paddy fields in Kerala have been facing two cardinal challenges, intensification and abandonment, besides the global climate change. Intensive agriculture practices have triggered loss of some ecosystem services and have exerted a range of negative impacts on the environment. The abandonment of paddy lands of the state has not only affected grain production, but also affected several ecosystem services that these unique ecosystems provide. Fallowing may have probably negative as well as positive impact on ecosystems5.
Microorganisms are widely distributed in wetland soils and have far reaching effects on the biological and geochemical environment. The soil microbes play a major role in modifying the properties of soil by catalysing the chemical reactions take place within the substratum. The environmental conditions of rice fields are less stable and there may be some shifts in microbial community structures for various reasons 6, 7. Soil microbial status provides basic function of rice field’s soil ecosystem8. Moreover, information on the quality and quantity of bacteria present in such environment are important in determining health of these ecosystems.
Kuttanad represents low-lying (0.5 to 2.5 m below mean sea level) deltaic rice growing tract of Kerala, spread over Alappuzha and Kottayam districts (between 9° 8´ N and 9° 52´ N latitudes and 76° 19´ E and 76° 44´ E longitudes). It is part of the Vembanad wetland ecosystem, the largest brackish water Ramsar site of the state in the south-western tip of India. Though several studies have been conducted for exploring the ecological perspectives of the rice fields of Kerala, no systematic studies have been made on microbial characterisation of these environment, particularly in context of the increasing tendency of the area to keep rice fields fallow. This study was intended to characterise and compare the microbial communities in the context of farming and fallowing in the rice fields of Kuttanad, Kerala.
Materials and Methods
The Kumarakom village, sprawls over an area of 51.67 km2 is the south west part of Kuttanad wet land system of Kerala. Like any other ‘Kuttanadan’ villages, Kumarakom also witnessed vast changes in land use pattern and marked more than 50% drop in rice cropped area during the past few decades. Surface (0-15cm depth) soil samples were collected for the present study from two isolated rice fields at Kumarakom village, one from a field where paddy is being traditionally cultivated(n=6) and the other from a paddy field left uncultivated(n=6) for several years. Samples were collected during Nov-Dec 2021 after the rice harvesting in the cultivated field. Soil samples scooped out aseptically were transferred to sterilized polythene bags and brought to the laboratory in ice boxes for subsequent analysis. All the samples were then divided evenly into two parts: one part was employed in physicochemical analysis, and the other part was used to profile soil microbial community characteristics.
Serial dilution and plating technique on appropriate media was employed for the enumeration of the culturable microorganisms from the samples. Samples were analysed for total heterotrophic bacterial (THB) load which presented as number of colonies forming units (cfu) in 1 gram of sediment. The isolation of culturable micro flora was done on different media9 to differentiate the various functional groups present in the soil samples. Isolated colonies from each plate purified to characterize morphologically and physiologically and to identify up to species level following standard schemes10. Prepared cell lysates were also used as template for amplification of 16S rRNA genes and molecular identification of the strain was analysed using sequencing technologies and BLAST analysis.
The portion of the soil samples kept for physicochemical analysis, after air drying were sieved to < 2 mm and used to determine pH, electrical conductivity and organic carbon. Soil water suspension of 1:2 was used to measure pH using electronic pH meter and electrical conductivity in electrical conductivity cell. Wet digestion method of Walkley and Black11 was employed to determine the % of soil organic carbon.
Results and Discussion
Though microorganisms contribute only 0.5 per cent of the soil mass, their impact is higher in determining the soil properties and processes. Microorganisms mediate 80-90% of the processes occurring in soil. The existence of microorganisms in soil is decided on the existence of ambient conditions due to factors such as physicochemical characteristics of soil, the type of vegetation and the nutrient availability in the soil12, 13. Cultivated rice plots were found higher in microbial biomass than their fallowed counterparts in the study (Table 1). The microbial biomass in any environment is decided by growth supporting factors present there. In complex soil ecosystems such as of paddy fields the various agronomic practices affect directly or indirectly the spatio-temporal distribution and activity of the functional groups of microorganisms14, 15.
Agronomic practices influence the physical, chemical and biological characteristics of agricultural fields. Soils of cultivated fields were reported to promote the growth of bacterial community due to the improvement of the physical properties of the soil by tillage16. Conventional tillage process accelerates microbial growth in paddy fields as tillage improves the physical properties of the soil. Tillage operation stimulate microbial activity as it increases soil aeration and infiltration of water and better exposure of the degradable materials to the microbial community 17, 18. In addition to the improved abiotic soil properties due to field management and subsequent microbial growth in agriculture fields, the crop plants also contribute to the enrichment of microorganisms in the rhizosphere19, 20. Present study also revealed higher total heterotrophic bacterial density in cultivated rice fields (Table 1) compared to the fallowed fields. THB density (mean) in the cultivated rice fields was 6.9 x 105 cfug-1 while that in the fallowed fields was 4.8 x 105 cfug-1(Fig.1).
Table 1: Total bacterial density and soil characteristics (range of variation) at the study sites
| Parameter | Cultivated field | Fallowed field | ||
| Minimum | Maximum | Minimum | Maximum | |
| Bacteria in cfu g -1soil(x 105)
pH EC (ds m-1) OC (%) |
5.8
4.29 1.16 1.36 |
8.2
4.59 3.28 2.68 |
3.8
3.85 0.86 1.36 |
6.1
4.12 2.88 2.98 |
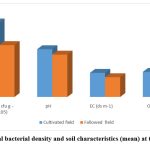 |
Figure 1: Total bacterial density and soil characteristics (mean) at the study sites |
Table 2: Bacterial species isolated with their incidence percentage in the study sites.
| Cultivated field | Fallowed field | ||
| Bacteria encountered | Percentage of incidence | Bacteria encountered | Percentage of incidence |
| Bacillus megaterium
Pseudomonas aeroginosa Klebsiella oxytoca Staphylococcus hominis Proteus mirabilis Bacteriodes fragilis Chromobacterium violaceum Bacillus cereus Escherichia coli |
100
100 100 80 60 60 60 40 30 |
Escherichia coli
Enterobacter aerogenes Citrobacter freundii Aeromonas hydrophila Acinetobacter baumanii Helicobacter pylori Yersinia tuberculosis |
100
80 80 60 60 40 40 |
Changes in the community structure of microorganisms is associated with the changes in physicochemical characteristics of soil21. All the samples recorded low pH and are found to be strongly acidic in nature. The uncultivated soil recorded the lowest pH of 3.85 (Table 1). Intermittent submergence and aeration can cause extreme acidic conditions as aeration of the soil leads to oxidation of sulphides to sulphuric acid22. An extreme low pH can reduce the availability of major and secondary nutrients in the soil. Electrical conductivity of the soil was found to be very high in all the samples (> 0.86) which might have been contributed by the soluble salts brought in by estuarine water. Organic carbon content of soils is an important soil quality indicator23, 24. Proportion of organic matter in soils vary with changes in land use pattern. The uncultivated soil had a higher organic carbon content compared with cultivated soils. Intensive rice culture causes a decrease in soil organic carbon content (mean=2.32 %) due to its increased utilization. Higher soil organic carbon content were reported from the abandoned field (mean= 2.45 %) where the soil is not ploughed and the land surface is covered with weeds and their residues.
The bacterial community identified from the study sites was composed of species that are commonly present in different types of soils. A total of 15 bacterial species were isolated in the study, 8 distinct from the cultivated field, 6 distinct from the fallowed field and 1 common to both fields (Table 2). Community structure and composition of microorganisms are greatly altered by changes in various biotic and abiotic factors of the particular environment. Differences in soil utilization pattern causes differences in the composition of the population of microorganisms inhabiting the soil25, 26. Fallowing has produced substantial changes in the soil micro biota from that of the cultivated field. The compositions of bacterial community in soils from uncultivated fields are entirely shifted from field where rice has been cultivated for extended periods of time. The highest frequency of occurrence (100 %) among cultivated field isolates were of Bacillus megaterium, Pseudomonas aeroginosa and Klebsiella oxytoca. Bacillus spp. are generally found in soils and play an important role in nitrogen cycle contributing to the fertility of soil27. Antibiotics that block harmful microorganisms are produced by some microorganisms. Bacillus megaterium has the capacity to produce antibiotics and to control several agricultural pests and diseases in addition to its plant growth promoting activity28, 29. Klebsiella spp.is generally abundant in soil, water, plant and animals and have major role in nitrogen fixation30. Pseudomonas spp. compete for nutrients with pathogenic microorganisms, hence considered beneficial to control most root pathogens present in soil.
Fertility of soils and sustainable crop production are maintained by the microbial community of the habitat. The soil micro flora compete with the saprophytic soil-borne pathogens for food and space which results in general disease suppression31. Disease suppressiveness is an outcome of diverse biological community and their collective interactions in the soil. Characterization of microbial population in the uncultivated sediment revealed the persistent occurrence of E. coli in the area along with higher incidence of Enterobacter aerogenes and Citrobacter freundii. Higher incidence of opportunistic pathogens Aeromonas sp., Helicobacter sp., Yersinia sp. etc.were also encountered in the fallowed field. Continuous rice cultivation under flooded conditions normally shifts the soil micro biota in a rice field towards a more consistent, plant growth promoting microbial community32.
The ecological stress on a habitat is directly revealed by the qualitative and quantitative status of its microbial components. The agricultural activity improves the quality of soil, hence the qualitative and quantitative composition of soil microorganisms. Cultivation processes positively influence the physical conditions of the soil, improves soil aeration that enhances infiltration of water and the biodegradable materials gets better exposure to the microbial biomass. The input of organic wastes in the cultivated field and its efficient decomposition ensures the availability of better environment for the growth of microorganisms. Due to fallowing, both quantitative as well as qualitative changes in microbial community, including losses in total bacterial biomass and diversity. Altered composition and density of bacterial community may directly or indirectly affect the biological activity of soil in the fallowed field.
Conclusion
In conclusion, the study showed that the bacterial community is distinct in cultivated and uncultivated rice fields. The increased bacterial diversity and density in the cultivated field may contribute to improved quality of soil and the habitat. Results of the study shows that fallowing alters soil pH and other soil properties followed by compositional as well as quantitative changes in soil bacteria of rice fields. Considering the important role of bacteria in maintaining the health of a habitat, it has been confirmed that soil quality deteriorates over long-term fallowing.
Acknowledgment
The authors are grateful to the Cholera and Biofilm research Lab, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram for the identification bacterial isolates.
Funding Sources
The present study receives no funding.
Conflicts of Interest
There is no conflict of interest.
Data Availability Statement
The manuscript incorporates all datasets produced in the study.
Ethics Approval Statement
The study does not involves any experiment on humans or animals.
Authors’ Contribution
1st author (Arjun S.) carried out the research work and prepared the manuscript.
2nd author (Dr. Anila Kumary K.S.) provided guidance for the study and made necessary corrections in the manuscript.
References
- Pimentel D., Wilson C., McCollum C., Huang R., Dwen P., Flack J., Tran Q., Saltman T. and Cliff B. Economic and environmental benefits of biodiversity. Bioscience. 1997; 47 (11):747-757.
CrossRef - Bjorklund J., Karin E.L. and Torbjorn R. Impact of production intensity on the ability of the agricultural landscapes to generate ecosystem services: an example from Sweden. Econ. 1999; 29:269-291.
CrossRef - Natuhara Y. Ecosystem services by paddy fields as substitutes of natural wetlands in Japan. Ecol.Eng. 2013; 56:97-106.
CrossRef - Nayak A.K., Shahid M., Nayak A.D., Dhal B., Moharana K.C., Mondal B., Tripathy R., Mohapatra S.D., Bhattacharyya P. Jambhulkar N.N., Shukla A.K., Fitton N., Smith P. and Pathak H. Assessment of ecosystem services of rice farms in eastern India. Ecol. Process. 2019; 8:35.
CrossRef - Mohankumar B. and Kunhamu T.K. Ecological and historical perspectives of rice cultivation in Kerala: a synthesis. Oryza. 2021; 58(2):24-261.
CrossRef - Prescott L.M., Harley J.P. and Klein D.N. 4th Edition. The McGraw Hill companies, Inc.NewYork 1999; 685pp.
- Pittol M., Scully E., Miller D., Durso L., Fiuza M. and Valiati V.H. Bacterial community of the rice flood water using cultivation- independent approaches. International Journal of Microbiology. 2018; ID 6280484.13pp.
CrossRef - Benila Smilya J. M., Vinoy Jacob and Ravi Kumar M. Soil micro flora of paddy fields among different rice farming system. J. Acad. Indus. Res. 2012; 1(1): 50-54.
- Seeley H.W. and Van Demark P.S. Microbes in action- A laboratory manual for microbiology. Freeman and Co. SanFrancisco, US. 1981; 388pp
- Buchanan, R.E. and Gibbons N.E. (ed.). Bergey’s manual of determinative bacteriology, 8th Ed. The Williams & Wilkins Co., Baltimore. 1979.
- Walkley, A. and Black, I. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934; 37(1): 29-38.
CrossRef - Girvan M.S, Bullimore J., Pretty J.N., Osborn A.M. and Ball A.S. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Applied Environmental Microbiology. 2003; 69:1800-1809.
CrossRef - Breidenbach B., Blaser M. B., Klose M. and Conrad R. Crop rotation of flooded rice with upland maize impacts the resident and active methanogenic microbial community. Environmental Microbiology, 2016; 18(9): 2868–2885.
CrossRef - Solante E.P. and Ohguchi T. Bacterial micro flora in cultivated and uncultivated rice fields and their effects on rice fungal pathogens in vitro. Pakistan Journal of Biological Sciences. 2000; 3(4):571-575.
CrossRef - Liesack W., Schnell S. and Revsbech N.P. Microbiology of flooded paddy fields. FEMSMicrobiol. Rev. 2000; 24:625-645.
CrossRef - Furtak K. and Gaida A.M. Activity and variety of soil microorganisms depending on the diversity of the soil tillage system. In Sustainability of Agro ecosystems Edited by A.B.de Oliveira.
CrossRef - Paul, E.A. and Clark F.E. Influences of management practices on soil organisms. In: Soil Microbiology and Biochemistry, Paul, E.A. and F.E. Clark (Eds.). Academic Press, London, 1989; pp: 87-88.
- Kladivko E.J. Tillage systems and soil ecology. Soil and Tillage Research. 2001; 61:61-76.
CrossRef - Edwards J., Johnson C., Santos-Medellín C., Lurie E., Podishetty N., Bhatnagar S., Elesen J. and Sundaresan V. Structure, variation and assembly of the root associated microbes in rice. Proc.Natl.Acad.Sci.USA. 2015; 112(8) E 911-20.
CrossRef - Hu L., Robert CAM, Cadot S., Zhang X., Ye M. and Li B. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere micro biota. Nat.Commun. 2018; 9 (1):2738.
CrossRef - Siu, , Zhang, R., Frey, B., Yang, L., Liu, Y., Ni, H., and Li, M. Soil physicochemical properties drive the variation in soil microbial communities along a forest successional series in a degraded wetland in north-eastern China. Ecol. Evol. 2021; 11: 2194-2208.
CrossRef - Beena S. George, Ashique, T.K. and Binitha, N.K. Assessment of microbial properties of pokkali soil in Kerala, India. Int.J.Curr.Microbiol.App.Sci. 2017; 6(12): 1964-1967.
CrossRef - Sparling G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Australian Journal of Soil Research. 1992; 30 (2):195-207.
CrossRef - Jacobs A., Rauber R. and Ludwig B. Soil and Tillage Research. 102:158-164.
CrossRef - Birkhofer K, Schoning I., Alt F., Herold N. and Klamer B. General relationships between abiotic soil properties and soil biota across spatial scales and different land-use types. PLoS One. 2012; 7(8):e43292.
CrossRef - Perera, T.A. and Tirimanne, S.Role of microbial communities in sustainable rice cultivation. In Role of Microbial Communities for Sustainability; Seneviratne, G., Zavahir, J.S., Eds.; Springer: Singapore; 2021; pp. 189–223. ISBN 978-981-15-9912-5.
CrossRef - Watanabe K. and Hayano K. Distribution and identification of proteolytic Bacillus spp. In paddy field soil under rice cultivation. Can.J. Microbiol. 1993; 39: 674-680.
CrossRef - Zhou Y., Choi Y.L., Sun M. and Yu Z. Novel roles of Bacillus thuriginesis to control plant diseases. Appl.Microbiol.Biotechnol. 2008; 80: 563-572.
CrossRef - Nguyen M., Ranamukhaarachchi S.L. and Hannaway D.B. Efficacy of antagonist strains of Bacillus megaterium, Enterobacter cloacae, Pichia guilliermondii and Candida ethanolica against bacterial wilt disease of tomato. J. Phytol., 2011; 3:1-10.
- Gelvin S.B. Agrobacterium mediated plant transformation – the biology behind the ockeying tool. Microbiol.Mol.Biol.Rev. 2003; 67(1): 16-37.
CrossRef - Berendesen R.L., Pieterse C.M. and Bakker P.A. The rhizisphere microbiome and plant health. Trends Plant Sci. 2012; 17(8):478-456.
CrossRef - Edwards J., Santos-Medellín C., Nguyen B., Kilmer J., Liechty Z., Veliz E., Jiadong Ni., Phillips G. and Sundaresan V. Soil domestication by rice cultivation result in plant- soil feedback through shifts in soil micro biota. Genome Biology. 2019; 20: 221.
CrossRef

