Introduction
In the last two to three decades, the bioactivity of phytochemicals has attracted increasing interest due to their potential use as insecticides against phytophagous insects1. Studies on phytochemicals and plant extracts have long been carried out to create pesticide substitutes that do not hurt human health and the environment. According to reports, pesticides derived from plants may alter the ratio of different biochemical elements in the body of an insect, alter the internal metabolism of the insect and lead to a reduction in activity or death. It is well known that these phytochemicals protect plants from insect pest attacks2. Insect feeding and oviposition on plants are influenced by phytochemicals produced in response to pest infestation. Phytochemicals have demonstrated various activities that can alter specific physiological processes in insects3-4-5. Pest and disease control has been attempted on thousands of plants. Currently, several horticultural mineral oils, vegetable oils, essential vegetable oils, and detergents are used worldwide to control pests and diseases6. Among all the biopesticides and plants that fight diseases, neem has proven to be the best choice.
Due to the presence of limonoid triterpenes7-8, the neem tree Azadirachta indica A.Juss (Meliaceae) has attracted attention as one of the most well-known biopesticides. Most of the research has been done on Azadirachtin, a limonoid found in the seeds of the Indian neem tree, A indica. Neem seed extract, rich in Azadirachtin, has strong anti-feeding and growth-regulating effects on insects9-10-11. Similarly, the limonoids present in Sentang (Azadirachta excelsa) are known to be effective feeding suppressants against a variety of pest species and have no adverse effects on beneficial insects, animals and humans12. Methanol preparations from A. excelsa wood were toxic to Crocidromia vinotalis larvae and impaired their development and feeding. In addition to a series of insect taxa from three different orders, Coleoptera, Lepidoptera, and Orthoptera, fruit extracts of A. indica showed feeding-deterrent activity against P. xylostella at higher doses14-15. Most phytophagous insects are effectively inhibited by Azadirachtin, but its effectiveness varies by species16. It has excellent anti-feeding properties against grasshoppers, the desert locusts.
Species most susceptible to neem extracts include butterflies17. It is well known that Azadirachtin has excellent activity against lepidopteran larvae18-19. Previous studies have shown that both topical and oral administration of these compounds to adult moths have long-term deleterious consequences, but neither substance is dangerous to adult moths in the short term20-22. Therefore, these sublethal effects are important to evaluate, as they may significantly impact the population dynamics of this lepidopteran pest and contribute to its control. This class of insecticides also contains several additional substances such as tebufenozide, halofenozide, and chromafenozide, all of which interact with the ecdysone receptor complex and cause premature and fatal molting, especially in caterpillars23-24.
Plumbagin and naphthoquinones, especially 5-hydroxy-1,4-naphthaquinone, are an important group of natural compounds that are very useful for the development of effective pesticides due to their abundance and relatively low toxicity. According to a literature review, Plumbagin from Plumbago zeylanica has many important biological effects, including antibacterial25, cytotoxic26, antimalarial27, antifilarial28 and antiprotozoal properties29. The naphthoquinone skeleton, with its abundant abundance and favourable structural properties, is an excellent model for producing effective feeding suppressants. It has previously been demonstrated that ingestion of crude aqueous methanol extracts of P. zeylanica and its derivatives results in failure of the moulting cycle in another lepidopteran insect, the silkworm (Bombyx mori). It has been shown to inhibit chitin formation and act as an insect repellent against a variety of lepidopteran pests30. The biological action of Plumbagin has been documented against two species namely D. koenigi and D. cingulatus31.
Koul32 pointed out that plant defences against insect herbivores usually never depend on a single component. Rather, many compounds interact with pests individually or together. Other biological effects on insects, such as larval growth inhibition, chronic toxicity, and prevention of oviposition, are also often associated with antifeeding effects33-34. In some situations, that period may be lost or long-term in nature35. The American bollworm H. armigera (Hubner) was exposed to the antifeeding effects and reduction of ecdysteroid titers by two well-known natural substances, Plumbagin from P. capensis and Azadirachtin from A. indica. This altered the activity of lysosomal enzymes and resulted in obvious morphological abnormalities during metamorphic molting36. Many studies have found that Azadirachtin and Plumbagin, which represent specific structural requirements for activity, are unchanged. Therefore, the authors of this study present the synthesis and evaluation of the antifeeding effects of Plumbagin and Azadirachtin on Pericallia ricini (Lepidoptera: Arctiidae).
Materials and Methods
Culture of the insects in the laboratory
P.ricini eggs and freshly emerged first-stage larvae were collected from castor plants grown near Madurai and stored at room temperature for the duration of the study (R.H). Fresh castor leaves were fed to the larvae were left to metamorphose into moths. The moths were treated with a sucrose (10% sucrose) solution soaked in small pieces of cotton. Males and females were housed in cages specially designed for mating. Laid eggs on the castor leaves put in the cage. Egg hatching was complete on the leaf. Fresh first-stage larvae were collected and separated from the eggs. These individuals were then stored in the laboratory.
Preparation of plant chemicals
Azadirachtin
Azadirachtin brand name Neemazal (5%) was obtained from the local market. 2.5 ml, 5 ml, 7.5 ml and 10 ml of azadirachtin were dissolved in 100 ml of distilled water separately to obtain concentrations of 25 ppm, 50 ppm, 75 ppm and 100 ppm for oral treatment. Similarly, for topical treatment, 1 ml, 2 ml and 3 ml of azadirachtin were dissolved in 10 ml of distilled water to obtain solution of 100 ppm, 200 ppm and 300 ppm concentration. 10 µL of the above solution was taken and applied topically to each larva.
Plumbagin
Plumbagin was procured from sigma company in Bangalore. Plumbagin 0.01ppm, 0.02ppm, 0.025ppm, 0.035ppm, 0.05ppm, 0.075ppm and 0.1ppm were dissolved in 1 ml of acetone. It has been used orally and topically.
Assessing larvicidal activity
For topical treatment, 50 µl of 0.01ppm, 0.02ppm, 0.025ppm, 0.035ppm, 0.05ppm, 0.075ppm and 0.1ppm of Plumbagin were applied topically to the newly formed fifth stage larvae P.ricini epidermis. . For oral treatment, fresh castor leaves were immersed in the test solution (control leaves in distilled water) for 30 seconds, drained and allowed to dry on filter paper for 30 minutes. Similarly, 10 µl of 100 ppm, 200 ppm, 300 ppm Azadirachtin were topically applied to the epidermis of newly emerged fifth instar larvae of P.ricini. For oral treatment, fresh castor leaves were immersed in a solution of 25 ppm, 50 ppm, 75 ppm and 100 ppm Azadirachtin for 30 seconds (control leaves in distilled water), drained and allowed to dry on filter paper for 30 minutes. At least twenty larvae per concentration were used in all experiments. And these experiments were repeated three times. Twenty-five larvae were placed in 250 ml plastic containers. For comparison, a separate control using acetone (1 µl per insect) was kept at the same time. Mortality at 24 h and 48 h was calculated for each concentration. The death rate was calculated37.


Results
Figure (1-4) summarize the insecticidal activity of two phytochemicals, namely Plumbagin and Azadirachtin, by both oral and topical administration. Plumbagin was administered orally and topically at concentrations of 0.01ppm, 0.02ppm, 0.025ppm, 0.035ppm, 0.05ppm, 0.075ppm and 0.1ppm. Similarly, oral Azadirachtin was administered at 25 ppm, 50 ppm, 75 ppm and 100 ppm and topical Azadirachtin was also administered at 100 ppm, 200 ppm and 300 ppm. Oral administration of Plumbagin causes statistically significant mortality in the larval stage.
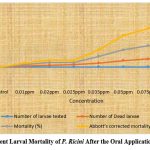 |
Figure 1: Per Cent Larval Mortality of P. Ricini After the Oral Application of Plumbagin. |
Mortality was lower with oral concentrations of Plumbagin 0.01ppm, 0.02ppm and 0.025ppm with mortality rates of 5%, 8.3% and 6.6%, respectively. Mortality was found to increase with increasing dose levels. For example, 0.035ppm Plumbagin caused 21.66% insect mortality and this treatment showed increased mortality at 0.050ppm, 0.075ppm and 0.1ppm. Those death rates were 35%, 43.33% and 53.33%. Similar results were obtained with topical treatment with Plumbagin. Topical treatment with Plumbagin showed 50% mortality at 0.1ppm and also 46.6% larval mortality at 0.075ppm Plumbagin. 41.6% larval mortality was significantly reduced at 0.05ppm Plumbagin concentration and correspondingly 30% larval mortality at 0.025ppm Plumbagin concentration figure (2).
 |
Figure 2: Per Cent Larval Mortality of P. Ricini After the Topical Application of Plumbagin. |
Subsequently, complete larval mortality was observed with 100 ppm oral Azadirachtin treatment and 80% larval mortality with 75 ppm oral Azadirachtin treatment. However, 50% larval mortality was observed at 50 ppm oral Azadirachtin treatment and 25% mortality was also observed at 25 ppm oral Azadirachtin treatment. Thus, no larval mortality was observed in control larvae (Figure 3) and 83.3% mortality was observed at 300 ppm with topical application. 71.6% larval mortality was observed with topical application of 200 ppm Azadirachtin and 61.6% larval mortality with 100 ppm Azadirachtin (Figure 4).
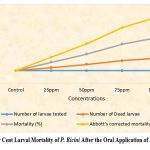 |
Figure 3: Per Cent Larval Mortality of P. Ricini After the Oral Application of Azadirachtin. |
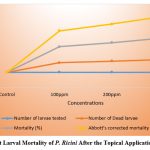 |
Figure 4: Per Cent Larval Mortality of P. Ricini After the Topical Application of Azadirachtin. |
Discussion
The present study highlighted that Plumbagin and Azadirachtin at different doses had inhibitory effects on the life cycles of P.ricini. As the concentration of Azadirachtin and Plumbagin increases at each stage of development, their toxicity also increases. Similar results have been observed and reported by several pest control workers38-39-40. The relative effectiveness of the doses of Plumbagin and Azadirachtin can be ranked based on the percentage of larval mortality at each dose level. The current study showed that two herbal compounds, Azadirachtin and Plumbagin, can be quite successful, although their effectiveness varies. At concentrations of 50 mg/ml, 75 mg/ml and 100 mg/ml, oral and topical administration of Plumbagin effectively reduced larval mortality. An increase in mortality was observed at a dose of 100 mg/ml of oral Plumbagin.
Similarly, two compounds related to plumbagin, juglone (5-hydroxy-1,4-naphthoquinone) and 2-hydroxy-1,4-naphthoquinone, showed inhibitory effects on insect ecdysis when tested at concentrations up to 2500 ppm30. Mulampurat et al41 also observed inhibition of feeding in Opisina arenocelis at higher doses (4 and 5 ug) of Plumbagin. Sumathy and Sanjayan42 also investigated the effect of Plumbagin on the post-ingestive effects of food consumption at concentrations of 25, 50, 100, 200, 400 and 800 ppm against third instar larvae of S. litura. Insects39, the inability to use the food they ate and digested caused a significant decline in individuals and increase in total mass. Banerjee et al.43 showed that plumbagin has growth regulating properties in insects. Plumbagin has been shown to have anti-feeding activity against many insect species in previous studies42.
When Plumbagin was applied topically, a concentration of 100 mg/ml caused 50% mortality. Therefore, the oral and topical concentrations of Plumbagin were determined to be 100 mg/mL, 75 mg/mL, and 50 mg/mL, respectively. Similar findings were reported by Villavicencio and Perez-Escandon44, who tested Plumbagin against Dactylotum coralllinum (Saussur) and Phoetaliotes nebrascencis (Thomas) and found it to have insect-eating properties. This larval mortality may be due to a decrease in chitin synthesis activity43. For proper ecdysis, high hemolymph pressure and muscle activity must be supported by a proper cuticle. Larvae with impaired nutrition, secretion or motility are the result of inhibition of ecdysis. Affected larvae are subsequently killed30 and as a result significant toxicity to P.ricini has been observed.
In addition, Plumbagin inhibits the chitin synthesis of Trichoplusia ni by about 30%. Pink bollworm had the lowest effective dose for 50% growth inhibition (ED50 = 150 ppm), while three other test species including Heliothis zea, H. virescens and T. ni (ED50 = 350 ppm) had comparable ED50 values. Because Plumbagin is unique to chitin-bearing insects, its insecticidal activity as an ecdysis inhibitor may make it more environmentally friendly than traditional neurotoxic insecticides. As a result, Plumbagin has been shown to be an effective toxic biopesticide against P. ricini and is a strong candidate for the title of “leading compound” in the study of synthetic pesticides.
Commercially available neem seed extracts have various pest control properties, including direct toxicity, anti-feeding and anti-oviposition effects, and effects on insect growth, fecundity and metamorphosis45. Lepidoptera exhibit strong antecedencies ranging from <1–50 ppm, contingent on the species, and are very susceptible to Azadirachtin46. Acetone leaf extract of A. indica was found to have anthelmintic activity against S. litura47. Osman16 found little variation in mortality of Pieris brassicae after treatment of 1-day-old fifth-instar larvae with 5.0 and 2.5% Azadirachtin. This study and the same toxic effect on P. ricini provide evidence of adverse effects of azadirachtin when used topically and orally. The present findings are supported by Mancebo et al.48 In studies, azatin, a neem seed extract containing 3% azadirachtin, was found to cause rapid direct toxicity against mahogany stem borer at relatively high concentrations (1.0, 3.20 and 10%). According to this experiment, total mortality was observed at 100 ppm oral Azadirachtin49.
According to a study by Richter et al.50 a commercial neem preparation containing 20% Azadirachtin, neemazal, has been shown to be effective in inhibiting the growth of the cockroach Periplaneta americana. It was observed in this experiment that mortality increased with increasing concentration. Similarly, Ahmed et al2 found that after 24 h treatment, T. castanium was killed by 18, 30, 52, 66 and 86% at 649.35, 974.0, 1298.7, 1623.37 and 1948.05 cm² concentrations of neem extract. Furthermore, within 48 hours of treatment, the neem preparation resulted in complete death of the European leafhopper Archips rosanus51.
The phytochemicals investigated in the present study – Plumbagin and Azadirachtin – all showed varying degrees of inhibition of feeding. Mortality of Plumbagin was 50% at 100 mg/ml oral and topical concentrations tested. At 50 ppm and 100 ppm oral and topical Azadirachtin treatment resulted in 50% mortality. Ulrichs and Mewis52 reported that mortality increased after a single treatment with a Neem product at doses of 0.01, 0.1, 0.2 and 1.0 g Azadirachtin/kg of rice.
In this investigation, the insecticidal activity of both Plumbagin and Azadirachtin was enhanced at concentrations of 100 ppm for Plumbagin applied topically and orally, and 50 ppm for oral and 100 ppm for topical application. According to the study and observations that showed that water loss increased with increasing doses of Plumbagin and Azadirachtin and mortality increased, this can be considered as one of the main causes of larval death. Thus, the introduction of compounds of plant origin can have a significant positive effect on the environment and the economy. In addition, these experiments showed that Plumbagin and Azadirachtin are more toxic to P.ricini.
Conclusion
The results of this study indicate that two plant compounds, Azadirachtin and Plumbagin, have the ability to suppress the larvicidal activity of specific lepidopteran agricultural pests, including Pericallia ricini and can be used as biopesticide against these pests. Thus, these biopesticides can play an important role in protecting environment against chemical pesticides
Acknowledgments
The authors are thankful to Dr. S.Dhanasekaran, Former Principal of Yadava College, Madurai and Yadava College management for providing all facilities and encouragement for the conduct of this work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
There is no conflict of interest.
Authors’ Contribution
Gnanamani. R- Lab work and framing the research paper
Ramanathan. B – Data Handling and framing the research work
Indira Rani. G– Correction and framing the Research paper
Ethics Approval Statement
No Ethical Issues
Data Availability Statement
None
References
- Padmaja, P.G. and Rao, P.J. Effect of plant oils on the haemolymph proteins of final instar larvae of Helicoverpa armigera Hubner. Entornon. 2000. 25(2):107-115.
CrossRef - Ahmad, M. Insecticide resistance mechanisms and their management in Helicoverpa armigera (Hübner), a review. J Agric Res.2007. 45: 319-335.
CrossRef - Liu, Y.Q., Yang, L. and Tian, X. Podophyllotoxin: current perspectives. Curr Bioact Compd. 2007. 3: 37-66.
CrossRef - Cantrell, C. L., Dayan, F. E., and Duke, S. O. Natural products as sources for new pesticides. J. Nat. Prod. 2012. 75, 1231–1242.
CrossRef - Hikal, W. M., Baeshen, R. S., and Said-Al, A. H. A. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017. 3:1404274.
CrossRef - Isman, M.B. “Botanical insecticides, deterrents, and repel-lents in modern agriculture and an increasingly regulated world,” Ann. Rev. Ent. 2006. vol. 51, pp. 45–66.
CrossRef - Aribi, N., Denis, B., Kilani-Morakchi, S., and Joly, D. L’azadirachtine, un pesticide naturel aux effets multiples. Medecine/Sciences. 2020. 36, 44–49.
CrossRef - Isman, M. B., and Grieneisen, M. L. Botanical insecticide research:many publications, limited useful data. Trends. Plant. Sci. 2014. 19,140–145.
CrossRef - Kraus, W. Azadirachtin and other triterpenoids. In: Schmutterer H (ed) Azadirachta indica A Juss and other meliaceous plants: sources of unique natural products for integrated pest management, medicine, industry and other purposes, 2nd edn. Neem Foun- dation, Mumbai, India. 2002. pp 39–110.
- Egwu, O. C., Dickson, M. A., Gabriel, O. T., Okai, I. R., and Amanabo, M. Risk assessment of heavy metals level in soil and jute leaves (Corchorus olitorius) treated with azadirachtin neem seed solution and organochlorine pesticides. Int. J. Envir. Agric. Biotech. 2019. 4, 756–766.
CrossRef - Isman, M.B., Matsuura, H., MacKinnon, S., Durst, T., Towers, G.H.N. and Arnason, J.T. Phytochemistry of the Meliaceae: so many terpenoids, so few insecticides. In: Romeo JT, Saunders JA, Barbosa P (Eds) Recent advances in phytochemistry, vol. 30. Phytochemical diversity and redundancy in ecological interactions. Plenum Press, New York, London. 1996. pp 155–178.
CrossRef - Mordue (Luntz), A.J. and Blackwell, A. Azadirachtin: An update. J. Insect Physol. 1993. 39: 903-924.
CrossRef - Ng, L.T., Yuen, P.M., Loke, W.H. and Kadir, A.A. Effects of Azadirachta extrcts on feeding behaviour, body weight, and mortality of Crocidolomia binotalis Zeller (Lepidoptera, Pyralidae). J Sci Food Agric. 2003. 83:1327–1330.
CrossRef - Charleston, D.S., Kfir, R. and Dicke, M. Behavior responses of diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) to extracts derived from Melia azedarach and Azadirachta indica. Bull Ento- mol Res. 2005. 95:457–469.
CrossRef - Carpinella, M.C., Defago, M.T., Valladares, G. and Palacios, S.M. Antifeedant and insecticidal properties of a limonoid from Melia Azedarach (Meliaceae) with potential use for pest management. J Agric Food Chem. 2003. 51:369–374.
CrossRef - Osman, M.Z. Effects of neem seed extract on growth and development of larvae of Pieris brassicae L. (Lep: Pieridae). J. Appl. Ent. 1993.115:254-258.
CrossRef - Schmutterer, H. and Singh, R.P. List of insect pests susceptible to neem products. pp. 326-365 in Schmutterer, H. (Ed.) The neem tree: source of uniaue natural products for integrated pest management, medicine, industry and other purposes. Weinheim, VCH.1996.
- Saenz-de-Cabezon, I.F.J., Marco, V., Salmo, F.G. and Perez-Moreno, L. Effects of methoxyfenozide on Lobesiabotana Den 8 Schiff (Lepidoptera: Torticidae) egg, larval and adult stages. Pest.Manag. Sci. 2005. 11:1133-1137.
CrossRef - Pineda, S., Schneider, M.I., Smagghe, G., Martinez, A.M., Estal, P.D., Vinula, E., Valle, J. and Budia, F. Lethal and sublethal effects of methoxyfenozide and spinosad on Spodoptera littoralis (Lepidoptera: Noctuidae).J. Econ. Entomol. 2007.100: 773-780.
CrossRef - Pineda, S., Smagghe, G., Schneider, M.I., Estal, P.D., Vinuela, E., Martinez, A.M. and Budia, F. Toxicity and pharmacokinetics of spinosad and methoxyfenozide to Spodoptera littoralis (Lepidoptera: Noctuidae) under laboratory conditions. Environ. Entomol. 2006. 35: 856- 864.
CrossRef - Grisakova, M., Metspalu, I., Jogar, K., Hiiesaar, K., kuusik, A. and Poldma, P. Effects of biopesticides neem EC on the large white butterfly, Pieris brassica L. (Lepidoptera: Noctuidae). Agron. Res. 2006. 4:181-186.
- Osorio, A., Martinez, A.M., Schneider, M.I., Diaz, O., Corrales, J.L., Aviles, M.C., Smagghe, G. and ineda, S. Monitoring of beet armyworm resistance to spinosad and methoxyfenozide in Mexico.Pest.Manag. Sci. 2008. 64: 1001-1007.
CrossRef - Dhadialla, T.S., Retnakaran, A. and Smagghe, G. Insect growth- and developmental-disturbing insecticides, in: Gilbert LI, Iatrou K, Gill SK (eds.), Compre. Mole. Insect Sci, Elsevier, Oxford. 2005. 6:55-116.
CrossRef - Yanagi, M., Tsukamoto, Y., Watanabe, T. and Kawagishi, A. Development of a novel lepidopteran insect control agent, chromafenozide. J. Pest.Sci. 2006. 31: 163-164.
CrossRef - Didry, N., Dubrevil, L. and Pinkas, M. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Die Pharmazie. 1994. 49(9): 681-683.
- Nguyen, A. T., Malonne, H., Duez, P., Vanhaelen-Fastre, R., Vanhaelen, M. and Fontaine, J. Cytotoxic constituents from Plumbago zeylanica. Fitoterapia. 2004. 75: 500–504.
CrossRef - Likhitwitayawuid, K., Kaewamatawong, R., Ruangrungsi, N. and Krungkrai, J. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Medica.1998. 64 (3): 237-241.
CrossRef - Mathew, N., Paily, K., Abidha, P., Kalyanasundaram, V. P. and Balamaram, M.K. Macrofilaricidal activity of the plant Plumbago indica/ rosea in vitro. Drug Develop. Res. 2002. 56(1): 33-39.
CrossRef - Kayser, O., Kiderlen, A. F., Laatsch, H. and Croft, S. L. In vitro leishmanicidal activity of monomeric and dimeric naphthoquinones. Acta Tropica. 2000. 76: 131–138.
CrossRef - Kuboa, I., Uchidaa, M. and Klocke, J.A. An Insect Ecdysis Inhibitor from the African Medicinal Plant, Plumbago capensis (Plumbaginaceae); a Naturally Occurring Chitin Synthetase Inhibitor. Agri & Bio. Che. 2014. 47 (4): 911.
CrossRef - Joshi, N.K., Lalitha, K.M., Banerji, A. and Chandha, M.S. Effects of Plumbagin on growth and development of red cotton bug Dystercus cingulatus Fab. Proc. Ind. Nat. Sci. Acad. 1998. 54: 43-46.
- Koul, O., Multani, J.S., Singh, G., Daniewski, W.M. and Berlozecki, S. 6b- Hydroxygedunin from Azadirachta indica, its potentiation effects with some non-azadirachtin limonoids in neem against lepidopteran larvae. J. Agri & Food Chem. 2003. 51: 2937-2942.
CrossRef - Pavela, R., Vrchotova, N. and Sera, B. Growth inhibitory effect of extracts from Reynoutria sp. Plants against Spodoptera littoralis larva. Agrociencia. 2008. 42: 573–584.
- Zapata, N., Budia, F., Vinuela, E. and Medina, P. Antifeedant and growth inhibitory effects of extracts and drimanes of Drimys winteri stem bark against Spodoptera littoralis (Lep., Noctuidae). Ind. Crop Prod. 2009. 30: 119–125.
CrossRef - Koul, O. Phytochemicals and insect control: an antifeedant approach. Crit. Rev. Plant Sci. 2008. 27: 1–24.
- Josephrajkumar, A., Subrahmanyam, B. and Srinivasan. Plumbagin and Azadirachtin deplete changes in the haemolymph ecdysteroid levels and enzyme profiles in the fat bodies of Helicoverpa armigera. Eur. J. Entomol. 1999. 96: 347–353.
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J Econ Entomol.1925.18: 265-7.
CrossRef - Jeyasankar, A., Premalatha, S. and. Elumalai, K. Antifeedant and insecticidal activities of selected plant extracts against Epilachna beetle, Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Ad. In. Ent. 2014. 2: 14-19.
CrossRef - Elumalai, T., Mathivanan, A., Elumalai , A., Jeyasankar, S., Dhanasekaran. and Krishnappa, K. Larvicidal and ovicidal properties of selected Indian medicinal plants extracts against American bollworm, Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae). Int. J. Inter. Res & Rev.. 2013. 1: 5-11.
- Adeyemi, M.M.H. The potential of secondary metabolites in plant material as deterrents against insect pests: A review. Afr. J. Pure& App. Che. 2010. 4: 243-246.
- Mulampurath, A. Suresh, K.and Asoke, B. Growth Disruption in Opisina arenosella walker by Plumbagin, a naturally occurring insect growth regulator, Insect Sci. Applic. 2002. 22(4): 321-323.
CrossRef - Sumathy, N. and Sanjayan, K.P. Effect of Plumbagin, a Napthoquinone of Plant Origin, on the Consumption and Post Ingestional Physiological Parameters of Food Utilization in Spodoptera litura (Fab) (Lepidoptera: Noctuidae). Glo. J.f App. Agri. Res. 2011. 1(2): 83-88.
- Banerjee, S., Magdum, G. P., Kalena. and Banerji, A. Insect growth regulatory activity of naturally occurring quinines and their derivatives in Dysdercus koenigii Fabr. (Hem., Pyrrhocoridae). J. Appl. Ent. 2001.125: 25-30.
CrossRef - Villavicencio, M. A. and Perez-Escandon, B. E. Plumbagin activity (from Plumbago pulchella Boiss. Plumbaginaceae) as a feeding deterrent for three species of Orthoptera. Folia Ent. Mexi. 1992. 86: 191-198.
- Naqvi, S.N.H. Prospects and development of a neem-based pesticide in Pakistan. Proc. Pakistan Congr. Zool. 1996.16: 325-338.
- Mordue (Luntz), A.J. and Nisbet, A.J. M. An Azadirachtin from the neem tree Azadirachta indica: its action against Insects. An. Soc. Entomol. Brasil. 2000. 29 (4), 615- 632.
CrossRef - Jeyarajan, S., Babu, P.C.S., Srimannarayana, G. and Geethanjali, Y. Antifeedant and morphogenetic effects of rich fractions on Spodoptera litura F. Bot. Pesticides Integr. Pest Manage. 1990. 13: 376-380
- Mancebo, F., Hilje, L., Mora, G.A. and Salazar, R. Biological activity of two neem (Azadirachta indica A. Juss., Meliaceae) products on Hypsipyla grandella (Lepidoptera: Pyralidae) larvae. Crop Prot. 2002. 21: 107-112.
CrossRef - Guerrini, V.H. and Kriticos, C.M. Effects of Azadirachtin on Ctenocephalides felis in the dog and the cat. Veter. Parasi. 1998. 74: 289-297.
CrossRef - Richter, K., Bohm, G.A. and Kleeberg, H. Effect of NeemAzal, a natural Azadirachtin -contaning preparation, on Periplaneta americana (L) (Orthopt, Blattidae). J. App. Ent-Zeit. Ange. Ent. 1997. 121: 59-64.
CrossRef - Aliniazee, M.T., Humeyri, A. and Saeed, M. Laboratory and field evaluation of a neem insecticide against Archips rosanus L. (Lepi: Tortricidae). Can. Ent.1997. 129:27-33.
CrossRef - Ulrichs, C. and Mewis, I. Controlling the stored product pest Sitophilus oryzae and Tribolium castaneum by contaminating rice with neem and diatomaceous earth. Anzeiger fur Schadlingskunde. 2000. 73 (2): 37-40.
CrossRef

