Introduction
Auxins were the first plant hormones to be identified. According to a study by Charles Darwin and his son Francis Darwin from 1880, “an issue that transmits its effects from one part of the plant to another” controls some plant development responses to plant growth. The verb “auxein,” from which this phrase is derived, meaning “to increase” or “to grow.” The most prevalent plant hormone of the auxin class, indole-3-acetic acid (IAA), controls various aspects of plant growth and development. As a result, the terms “auxin” and “IAA” are occasionally used synonymously. Despite IAA’s significance for plant development, little is known about the history of IAA biosynthesis and how natural selection shapes biosynthetic pathways. IAA can be produced by both plants and microscopic organisms, such as bacteria and fungi. The function of microbial IAA in interactions between plants and microbes has recently received more attention. The relationship between plants and phytopathogenic bacteria is well known, and it can prevent the growth of plants by upsetting the balance of auxin in the plants and generating tumours and galls. Additionally, a number of studies have demonstrated that IAA in microorganisms functions as a signalling molecule by influencing the expression of genes in various microbes. Interactions between IAA-producing organisms may be impacted by IAA. Between IAA-producing bacteria and plants, as well as between bacteria, the auxin functions as an effector molecule.1, 2
IAA in fungal- plant interactions
IAA’s physiological functions in plants are the important auxin in plants that regulate growth, development processes such as cell division, apical dominance, tissue differentiation, elongation, light, gravity and pathogens responses. The most susceptible tissues to changes in IAA level are roots. For different tissues, the primary and lateral roots begin at different times. IAA is required for both primary and lateral root initiation. IAA promotes lateral root production, adventitious root development and a dose-dependent lengthening of root hair. IAA also has an important influence on how the vascular network and leaf morphogenesis develop. IAA plays a role in pathogenic interactions with plants, including pathogenesis and defence mechanisms. The functions of fungal-produced IAA in different plant-fungal interaction systems imply that fungi may employ IAA and similar substances to connect with plants for pathogenic or symbiotic methods, promoting plant growth and altering the fundamental plant defence mechanism. The impacts of fungal-produced IAA on plant relationships, root growth, and development, as well as their rhizosphere-associated microorganisms, such as fungi, were of particular interest because understanding these processes could result in environmentally friendly agricultural practises. Numerous organic substances, including sugars, organic acids, and vitamins, are produced by roots. The colonies of fungi then utilise these as nutrition or signals. In contrast, the siderophores, volatile substances, and phytohormones released by fungi can either directly or indirectly promote plant development by increasing the availability of nutrients in their host. IAA generated by fungi can promote the growth of root hair and lateral roots. Plants nearby absorb more nutrients when root development and growth are encouraged. As a result, there is an increase in the biomass production of fruit and/or shooting. As a result, these IAA-producing fungi can be effectively employed as a substitute for synthetic fertilisers to enhance plant development. Furthermore, fungal- produced IAA can indirectly affect plants by strengthening the immune response of plants to suppress phytopathogenic strains and the development of disease.3, 4, 20, 21
Phyllanthus emblica Linn
Amla (Indian gooseberry) is a member of Euphorbiceae family also known as Emblica officinalis Gaertn. This is an important plant in Ayurveda, Siddha, Unani and naturopathy medicine system. It has twice as much vitamin-C as lemon juice. It has 30 cases of polyphenols greater than violet wine and has more gallic acid (a powerful antioxidant) than other fruit. Parts of the plant used in traditional Indian medicine are the fruits, seeds, leaves, root, bark, and flowers.5-7
Materials and Methods
Plant collection
The plant sample of Phyllanthus emblica Linn. was collected from the Veer Narmad South Gujarat University Hostel, Vesu, Surat, Gujarat, India.
Selection of plants and Isolation of Endophytic Fungi
Healthy and mature leaf and stem samples were selected for the isolation of fungi. The samples initially subjected for surface sterilization as per the standard methodology given with some modification.8 Plant part washed with running tap water. Then leaf and stem cut small pieces. The samples were surface sanitized with 70% alcohol for 3 minutes and 4% Sodium hypochloride for 2 minutes. Again washed with 70% alcohol for 1 minute. Last washed with distilled water and blot dried on sterile tissue paper. That segments to inoculated plate containing potato dextrose media with antibiotics Chloramphenicol (50 mg/L). All the plate incubated at 28○C for 7 to 10 days.9, 10
Identification of endophytic fungi
Endophytes fungi were observed morphologically and microscopically. The microscopically observed by mounting. To take a small fungal mycelia on PDA plates. And strain with lacto phenol picric acid and observed in light microscope (40X). That structure identified with the help of standard manual. The morphologically observation on the basis of colony diameter, texture, colour and morphology of hyphae and conidia.11, 12
Isolate screening for IAA production
IAA production was estimated using Patten and Glick’s (2002) methodology with a little modification. L-tryptophan solution was used to inoculate Aspergillus sp. spores into Czpeak Dox Broth, which was then kept at room temperature for 10 days at 28 oC. The culture was centrifuged at 5500 rpm for 15 minutes following a 10-day incubation period. Salkowaski reagent was combined with 1 ml of supernatant and 2 ml of it was then incubated for 30 minutes in the dark. Pink colour development suggested IAA production. IAA was quantified using a UV-Vis spectrophotometer at 530 nm. The IAA solution was qualified using a standard curve. The amount of IAA in the culture was expressed as mg/ml.13
Parameter optimisation for IAA production
In this work, the generation of IAA was optimised for a selected isolate Aspergillus strain by adjusting one parameter at a time.14, 15
L-tryptophan concentration effect
Using Czpeak dox broth supplemented with L-tryptophan concentrations of 1, 2, and 5 mg/ml and without tryptophan, the effect of L-tryptophan concentrations on IAA synthesis was examined. The culture was incubated at room temperature for 10 days.
Effect of incubations time
The effects of incubation time on the generation of IAA by Aspergillus sp. were studied after the mould was grown in 20 ml of Czpeak dox broth supplemented with 5 mg/ml L-tryptophan and incubated at room temperature for 10 days. IAA production by allowing an Aspergillus sp. culture to grow for up to 10 days in ideal conditions.
Selection of culture media
IAA production by Aspergillus sp. was optimized with different broth such as Salt- basal medium, Potato dextrose broth and Czpeak dox broth. The production of IAA was observed after 9 days of incubation at room temperature.16
Crude IAA extraction
According to Shanmugaiah et al. (2010), the standard solvent extraction approach was used to extract Indole acetic acid from Aspergillus sp. Centrifugation at 5500 rpm for 20 minutes was used to separate the cells from the supernatants after an incubation period of 10 days. The supernatant was extracted in double volume with ethyl acetate after being acidified with 1 N HCl to pH 2. A separating funnel was used to separate the extracted ethyl acetate portion. That layer separate after dried in Petri plates in open environment. After the white crystal dissolved in small amount of methanol and kept at -20○C.17
Results and Discussions
Isolation of Endophytic Fungi
The plant samples were initially for surface sterilization as per the standard methodology along with some modification.8 After sterilization that plant sample was dried and inoculation on Potato dextrose agar (PDA) plates. That plates incubation at room temperature for 7 to 8 days.
Identification of Fungi (Microscopic)
Endophytic fungi were observed morphologically and microscopically. The microscopically observed by mounting. To take a small fungal mycelia on PDA plates and strain with lacto phenol picric acid and observed in light microscope (40X). That structure identified with the help of standard manual (Fig. 1).
 |
Figure 1: Structure of different strains of Endophytic Fungi. |
Identification based on morphological characteristics
The morphologically observation on the basis of colony diameter, texture, colour and morphology of hyphae and conidia18 (Table 1).
Table 1: Identification based on Colony morphology and Morphological characteristics
| Fungi isolated | Colony Morphology | Morphological Characteristics |
| Strain 1
(Aspergillu fumigatus) |
Blue green colour colony growing fast covering the disk, in 5-7 days at 37○C. | They generate spores with a diameter of 200 to 400 µm. Around the apex, the stipes are grey in colour. They have a slick exterior. Resembling a tiny, columnar globus. Conidia can have a smooth or spinose surface. |
|
Strain 2 (Aspergillus ustus)
|
A. ustus colonies have a dull brown, purplish to grey brown, or dark brown exterior with a yellow to brown interior. |
They produce spores that range in size from 700 to 2500 µm. Around the apex, the stipes are grey in colour. They have a smooth appearance. In them is mycelium. |
| Strain 3
(Aspergillus oryzae) |
Bluish to green colour, powdery like spores. | They produce spore between 500 and 2500 µm. The stipes is uncolored. They have a rough surface. The shape of globuse. The surface of the conidia is either smooth. |
| Strain 4
(Aspergillus flavus) |
velvety , yellow to green or brown |
They produce spores range of 400 and 800 µm. The stipes is pale brown. They have a quietly spherical surface. The shape of a globuse ellipsoid. The conidia is smooth finely roughened. |
Optimization of parameters
Effect of L-tryptophan
Using 20 mL of Czpeak dox broth supplemented with L-tryptophan concentrations of 1, 2, and 5 mg/mL and without tryptophan, the effect of L-tryptophan concentrations on IAA synthesis was studied. The culture was grown for ten days at room temperature.
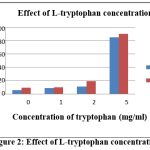 |
Figure 2: Effect of L-tryptophan concentration |
After 10 days incubations, culture was centrifuge at 5500 rpm for 14-16 minutes. The amount of IAA was measured at 530 nm in Ultra Violet Vis spectrophotometer. The standard curve were plotted for qualification of IAA. The spectrophotometric study revealed a progressive increase in IAA generation with increasing L-tryptophan content. However, 5 mg/ml L-tryptophan contents in the A. ustus showed highest production of IAA in Fig 2.
Effect of Incubation period
Aspergillus sp. was cultured in 20 ml of Czpeak dox broth supplemented with 5 mg/ml L-tryptophan and incubated at room temperature for up to 10 days to examine the effects of incubation period on IAA production.
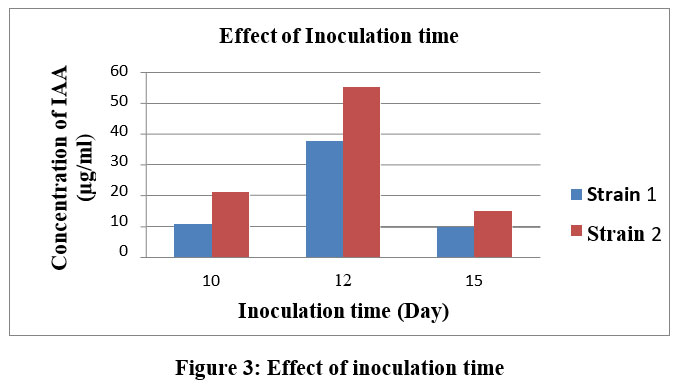 |
Figure 3: Effect of inoculation time |
After 10 days incubations, the culture was centrifuged at 5500 rpm for 14-16 minutes. IAA was determined using an Ultra Violet Vis-spectrophotometer at wavelength 530 nm. The IAA solution was validated using a standard curve. The spectrophotometric study showed that IAA production gradually increased over a period of up to 10 days, with A. ustus producing the most IAA after 12 days of incubation. After 12 day the decrease amount of IAA in the medium (Fig. 3).
Effect of different Culture media
Different broth, such as Salt-basal medium (broth), Potato dextrose broth, and Czpeak dox broth, were used to optimise IAA synthesis by Aspergillus sp. After 10 days of incubation at room temperature, IAA production was seen. The culture was centrifuged at 5500 rpm for 15 minutes after being incubated for 10 days. IAA was evaluated using a UV-Vis spectrophotometer at 530 nm. The IAA solution was validated using a standard curve.
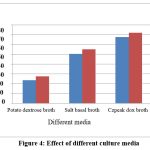 |
Figure 4: Effect of different culture media |
In a similar manner, A. ustus in Czpeak dox broth produced the most IAA out of the three media tested. In contrast, PDA medium showed the least amount of IAA formation when compared to control (Fig. 4).
Extraction of crude IAA
Extraction of crude IAA: Using the customary solvent extraction method, indole acetic acid was obtained from Aspergillus sp. using as standard methods17 (Fig. 5).
 |
Figure 5: Extraction of crude IAA white colour crystals (IAA). |
 |
Figure 6: Drying IAA crystals dissolved in methanol. |
 |
Figure 7: Salkowaski test |
After 10 days of incubation period, cells were separated from the supernatants by centrifugation at 5500 rpm for 20 minutes. The supernatant was extracted in double volume with ethyl acetate after being acidified to pH 2 with 1 N HCl. The extracted ethyl acetate fraction was separated by separating funnel. That layer separate after dried in Petri plates in open environment. After the white crystal dissolved in small amount of methanol and kept at -20○C (Fig. 6). The quantification of IAA was measured at 530 nm in UV-Vis-spectrophotometer using Salkowaski test19 (Fig. 7).
The IAA production of all four isolates was positive, however only those two isolates were chosen as probable IAA producers. IAA generation by fungal strains has reportedly been impacted by substrate availability, development stage, and culture conditions. An important option for a qualitative determination was the employment of the Van Urk-Salkowski reagent for the detection of IAA. The amount of IAA generated by the fungi was below Salkowaski reagent’s detection threshold. L-tryptophan does not interact with the reagent, which reacts with IAA. Isolated A. ustus is one of the top IAA producers. Therefore, these isolates were chosen for further characterization.
For the synthesis of IAA, various concentrations of L-tryptophan were selected, ranging from 1 to 5 mg/ml. The spectrophotometric study showed that the corresponding L-tryptophan concentration was associated with a progressive rise in IAA generation. However, maximum IAA synthesis was observed at a medium L-tryptophan concentration of 5mg/ml. When compared to a standard graph, that production. The impact on IAA production was anticipated to last up to 10 days, with the maximum IAA production being shown in an incubation period of 15 days. Similarly, Czpeak dox broth showed the highest level of IAA generation among the three tested media. In contrast, PDA medium showed the least amount of IAA formation when compared to control. Indole acetic acid was extracted from Aspergillus species using the standard solvent extraction process. Salkowaski test was used to quantify the IAA using the UV-vis spectrophotometric method. The highest amount of IAA is produced by Aspergillus ustus (72 g/20 ml).
Conclusions
According to this results, it was clear that P. emblica plant can be a good source of Indole Acetic Acid producing fungi and have the capacity to create significant quantities of IAA in a tryptophan-supplemented medium. The total four isolates were found as IAA producing strains, from these two were media components producing IAA, and physical parameters were optimized for IAA synthesis. Among the strains isolates, the stem and leaf tissues of P. emblica show best growth promoting activity in A. ustus (72 μg/20 ml). The beneficial impacts on the growth of crops and yield are due to the presence of such growth-promoting fungi.
The potential for these isolates to produce IAA and the study on IAA production optimisation will increase the growth and ultimately, IAA production in the field and prevent environmental pollution by avoiding excessive applications of industrial fertilizers to cultivated fields, which is the significance of the study.
Acknowledgments
The authors wish to thank Head, Bio-technology Department, V. N. S. G. University, Udhna-magdalla road, Surat, Gujarat (India) and Head, Department of Botany, SSVP Sansthas, L. K. Dr. P. R. Ghogrey Science College, Dhule for providing the necessary laboratory facilities.
Conflict of Interest
There is no conflict of interest.
References
- Anjali Bose, Dharti Shah, Keharia H. Production of indole-3-acetic acid (IAA) by the white rot fungus Pleurotusostreatus under submerged condition of Jatropha seedcake. Mycology: International journal on Fungal Biology. 2013; 4(2): 103-111.
CrossRef - De Battista J P, Bacon CW, Severson R, Plattner RD, Bouton JH. Indole Acetic acid production by the fungal endophyte of tall Fescue. Agron J. 1990; 82: 878-880.
CrossRef - Nita Patil, Milind Gajbhiye, Aparna Gujanjal, Sangeeta A. Optimization of Indole acetic acid production by acetobacter diazotrophicus L1 isolated from sugarcane. Int J envi sci. 2011; 2(1): 454-475.
- Shih-Feng Fu, Jyuan-Yu Wei, Hung-Wei Chen, Yen-Yu Liu, Hsueh-Yu Lu, and Jui-Yu C. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signaling & Behavior. 2015; 10:
CrossRef - Dhale Pharmacognostic Evaluation of Phyllanthus emblica Linn. International Journal of Pharma and Bio Sciences. 2012; 3(3): 210-217.
- Dhale DA, Mogle UP. Phytochemical Screening and Antibacterial Activity of Phyllanthus emblica (L.). Science Research Reporter. 2011; 1(3): 138-142.
- Dhale DA. Medicinal Herbs for Pharma Industry.P.S. Publishing House, Darya Ganj, New Delhi, India, 2022.
- Hallmann J, Berg G, Schulz B. Isolation procedures for endophytic microorganisms. Springer, Berlin Heildberg. 2007; 4 (1): 299.
CrossRef - Rodriguez RJ, White JF, Arnold AE. Fungal endophytes: diversity and functional roles. New Phytol. 2009; 182: 314-330.
CrossRef - Pandey Pramod Kuma, Singh Siddhartha, Yadav Raj Naraian Singh, Singh Amit Kumar, Singh M, Chandra K. Fungal endophytes: promising tools for pharmaceutical science. International Journal of Pharmaceutical Sciences Review & Research. 2014; 25: 128–138.
- Madhurama Gangwar, Navneet Kaur, Preeti Saini A. The diversity, plant growth promoting and anti-microbial activities of endophytic actinomycetes isolated from Emblica officinalis Int J Adv Res. 2015; 3(4): 1062-1071.
- Maheshwari Rajamanikyam, Varahalarao Vadlapudi, Ramarsamanchy, Suryanarayana Murt U. Endophytic fungi as novel resources of natural therapeutics. Braz Arch boil technol. 2017; 4 (1): 60.
CrossRef - Patten LC, Glick RB. Role of Pseudomonas putida Indole acetic acid in development of the host plant root system. Appl Environ Microbiol. 2002; 68: 3795-3801.
CrossRef - Hariharan Harikrishnan, Vellasamy Shanmugaiah, Natesan B. Optimization for production of Indole acetic acid (IAA) by plant growth promoting Streptomyces sp isolated from rice rhizosphere. Int J Curr Microbiol App Sci. 2014: 3(8): 158-171.
- Nita patil, Milind Gajbhiye, Aparna Gujanjal, Ahiwale S. Optimization of Indole acetic acid production by acetobacter diazotrophicus L1 isolated from sugarcane. Int J envi sci. 2011; 2(1):454-475.
- Hallmann J, Berg G, Schulz B. Isolation procedures for endophytic microorganisms. Springer, Berlin Heildberg. 2007; 4 (1): 299.
CrossRef - Shanmugaiah V, Mathivanan N, Varghese B. Purification, crystal structure and antimicrobial activity of phenazine-1-carboxamide produced by a growth-promoting biocontrol bacterium, Pseudomonas aeruginosa. Journal of Applied Microbiology. 2010; 108: 703-711.
CrossRef - Diba KP, Kordbacheh SH, Mirhendi SR. Identification of Aspergillus species using morphological characteristics. Pak J Med Sci. 2007; 23 (6): 867-872.
- Rahman Atiqur, Sitepu Irnayuli R, Tang Sui-Yan, Hashidoko Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil.Bioscience, Biotechnology and Biochemistry, 2010; 74 (11): 2202–2208.
CrossRef - Fu SF, Wei JY, Chen HW, Liu YY, Lu HY, Chou JY. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal Behav. 2015; 10(8):e1048052. doi: 10.1080/15592324.2015.1048052. PMID: 26179718; PMCID: PMC4623019.
CrossRef - Zhang BX, Li PS, Wang YY, Wang JJ, Liu XL, Wang XY, Hu XM. Characterization and synthesis of indole-3-acetic acid in plant growth promoting Enterobacter RSC Adv. 2021 Sep 24; 11(50):31601-31607. doi: 10.1039/d1ra05659j. PMID: 35496854; PMCID: PMC9041686.
CrossRef

