Introduction
Rice (Oryza sativa L.) is a primary source of food for one third of the world’s population and cited as a most important cereal crop1 belongs to family Poaceae having chromosome no 2n= 24. 2 Two main cultivated species of Oryza genus are Oryza sativa (Asian rice) and Oryza glaberrima (African rice),3 As a diploid crop and a relatively small genome 430 Mb,4 rice holds a great potential for understanding genetic mechanisms of crop domestication and improvement. This crop is also riched in amino acids like lysine, tryptophan and vitamins such as vitamin B1, vitamin B2 and folic acid and also act as a good source of minerals iron, zinc, calcium, phosphorus and selenium present in black rice that makes it highly nutritious for health. Phytochemical constituents include carotenoids, phenolics, alkaloids, nitrogen and organosulfur containing compounds and among antioxidants, Phenolics plays a significant role which further categorized as phenolic acids, flavonoids, coumarins and tannins.5, 6, 7
India is enriched with a greatest number of short and slender aromatic rice varieties that are famous in traditional rice growing regions. These unique rice germplasms have immense potential of containing valuable genes that can be effectively utilized in present day breeding program not only to explore high yield potential and quality but also resistant to biotic and abiotic stresses.8 As compared to other Asian countries, aroma qualities of Indian aromatic rice varieties have a special feature that enhances the value of rice in international market.9 As a consequence, rice needs a special attention with respect to its cooking quality as well as some biochemical and morphological characteristics.10 Although coloured rice is less consumed, there are many special rice varieties that contain coloured pigments, such as black, brown and red owing to functional effects. Among them black rice’ belongs to species Oryza sativa, and is predominantly famous for its purple colour of grain due to presence of high amount of anthocyanins in the aleurone layer.11 In particular, presence of anthocyanins is associated with health effects such as dietary antioxidants, anti-inflammatory compounds and hypoglycemic activities.12
Utilization of Ionizing radiations through induced mutagenesis has played an important role in the crop improvement. Determination of optimum dose, radio-sensitivity and treatment conditions are most essential for genetic manipulation through induced mutation. Gamma rays belong to ionizing radiation and interact with atoms or molecules to produce free radicals in cells that can damage or modify important components of plant cells and have been reported to effect differentially the morphology, anatomy, biochemistry and physiology of plants depending on the irradiation level. These effects include changes in plant cellular structure and metabolism e.g. dilation of thylakoid membranes, alteration of photosynthesis, modulation of antioxidant system and accumulation of phenolics.13,14,15
Previous studies reported, that the exposure of gamma irradiation of 0.1 KGy act against biotic stress and exposure to low doses of 0.5 KGy to suitable for enhancement of aroma in aromatic rice.16 Exposure to gamma rays from 200 Gy to 400 Gy induces chlorophyll concentration in normal rice.17,18 Exposure to 2.5 KGy and 5 KGy of gamma irradiation responsible for increment in sugar and antioxidant activities in rice. So, the purpose of the present study was to subject the seeds of black rice to different doses of gamma rays and search for desirable variations in biochemical and physiological characteristics in plants that may be helpful in further insight into the development of quality traits for future plant breeding programs.
Materials and methods
Plant material
The present study was conducted using gamma irradiated seeds of black rice collected from a local farmer which were cultivated in the fields of The Agricultural and Food Engineering Department (Plot size = 5m2), Indian Institute of Technology, Kharagpur, West Bengal, India. Seeds without any treatment were sowed as control. Rice seeds were irradiated using gamma radiation facility in gamma irradiation chamber GC 1200 (Board of Radiation and Isotope Technology, BRIT) with Co60 isotope. Samples were exposed to gamma irradiation doses of 50 Gy, 100 Gy, 150 Gy and 200 Gy with a dose rate of 2.65 KGy (kilo gray) per hour at UGC- DAE consortium for scientific research council, Kolkata, West Bengal, India. After 23 days of seeding, transplantation was done following a random block design pattern with plant to plant and row to row distance of 0.25 m. Third leaf from each irradiated plants were collected after the reproductive stage and three replications from each doses used for biochemical and physiological analysis.
Biochemical and physiological analysis
The leaves collected from cultivated plants were used for non-enzymatic antioxidant estimation. For estimation of total phenolics methods of19 were followed. Total flavonoid content was measured by AlCl3 method of 20 as earlier described.21 Methods of 22 was followed for estimation of total carotenoids. Lipid peroxidation was measured by the method of 23 to see the effect of irradiation on membrane. Total proline was measured by the method of 24 to check the water stress that induces metabolic irregularities in plants. For estimation of reactive oxygen species level, Superoxide anion (O2 –) radical content was estimated using nitro blue tetrazolium (NBT) solution by methods of 20 as described earlier by25 Hydrogen peroxide (H2O2) was measured by methods of.26 Methods of 27 were used to estimation of total chlorophyll from third leaves to know the effect of irradiation in important plant pigments. Total soluble sugar was measured by the method of 28 from third leaves.
Specific activity of antioxidative enzymes peroxidase, catalase and superoxide dismutase were measured. In the present study, enzyme activities were represented as specific activity in microgram (U mg-1) of protein. For all the assays, enzymes were extracted by homogenizing 50 milligram(mg) fresh leaves in 100 (millimolar) mM chilled phosphate buffer (pH 7.0) containing 1 mM EDTA, 1mM phenylmethylsulphonyl fluoride (PMSF) and 1 % (w/v) polyvinylpyrrolidone (PVPP). For all the irradiated samples total soluble protein contents of the plant samples were assayed according to Bradford method.29 Guaiacol oxidation method was followed for estimation of peroxidase activity.30 Catalase activity was measured according to31 as also described in32 NBT reduction method was followed for measuring SOD activity exactly the same way as described by.33
Statistical analysis
Analysis of Variance (TWO WAY ANNOVA) was done followed by POST-HOC Test, to know the variation among different doses at the statistical significance of p<0.05. The Pearson correlation statistics were performed between different biochemical and physiological attributes of irradiated doses to test the level of significance using Statistics SPSS16.0 software.
Results
Estimation of non-enzymatic antioxidants in leaves from different doses of gamma irradiated samples
In the present investigation, total phenolics, total flavonoids and total carotenoid content was measured to determine the non-enzymatic antioxidants accumulation in the leaves of plants grown from gamma irradiated seeds. The analysis of variance (TWO WAY ANOVA) followed by POST HOC analysis was done to indicate the effect of ionizing radiation among different doses with statistical significance at the level p˂ 0.05. Total flavonoid content (TFC) of leaves from different doses of gamma irradiated samples was expressed as mg quercetin equivalent per gram fresh weight. High amount of flavonoid was accumulated from 50 Gy (4.23±0.27) to 200 Gy (11.8±0.35) as compared to control (Figure 1A) and according to ANNOVA and post hoc analysis, a significant difference was seen from low to high doses at P < 0.05. Total phenolic content (TPC) of leaves was expressed in terms of µg (microgram) gallic acid equivalent per gram fresh weight. As compared to control, 50 Gy (56.93±1.9) sample, accumulated high phenolics and same trend was also followed for higher doses indicating , plants released a high amount of antioxidants to cope with exposure to high irradiation doses (Figure 1B). Total carotenoid accumulation has effectively increased with respect to increase in dosage, with higher doses accumulating significantly higher carotenoids (p˂ 0.05) (Figure 1C).
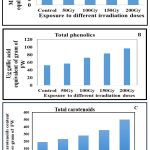 |
Figure 1: A Variation in flavonoid level in irradiated rice ; B Variation in phenolics level; C Variation in carotenoids level; Differenct doses of mean with variation are significantly different at p<0.05 level. |
Estimation of Stress Indicators
The level of Malon dialdehyde (MDA) produced during peroxidation of membrane was determined and MDA content has significantly increased with increasing dosage (Figure 2A) indicating 200 Gy (0.28± 0.015) of irradiation severely affects the membrane lipids in plant cell (significance at the level of p˂ 0.05). In the present study, proline concentration was measured from third leaf of seedlings to know the level of water stress and related metabolic irregularities. Proline concentration is showing significant variation (p ˂ 0.05) at different doses from low (30.05±0.63) to high doses (50.31±0.91)which was showing high proline concentration and activates both enzymatic and non-enzymatic anti-oxidative mechanism( correlation significant at 0.01 and 0.05 level). Variation in proline concentration in different doses shown in Figure 2B
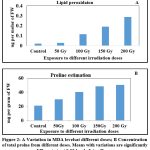 |
Figure 2: A Variation in MDA levelsat different doses; B Concentration of total prolne from different doses. Means with variations are significantly different at p< 0.05 level of significance. |
Estimation of reactive oxygen species
In this study concentration of H2O2 and O2 – were measured in terms of mg per gram of FW and µM per gram of FW. An inverse correlation was found between O2– (0.24± 0.014, Fig 3B) and H2O2 (6.44±1.99, Fig 3A) levels (Correlation significant at r = -0.718). In higher doses (200 Gy) accumulation of H2O2 (31.68±1.4) was higher as compared to O2–(0.10±0.005), (p< -0.718) indicates a possible regulation of ROS status in gamma irradiated plants by superoxide dismutase (SOD) enzyme.
 |
Figure 3: Represents status of ROS at different doses. A: Variation in H2O2 level; B: Variation in superoxide level. Means with variations are significantly different at P˂ 0.05 level of significance. |
Anti-oxidative enzyme activity estimation
The effect of irradiation on the activity of antioxidative enzymes was examined by measuring specific activity which defines the efficiency of an enzyme during conversion of substrate to products. Activity of three antioxidative enzymes was measured in terms of U-mg-1min-1. Activity of guaiacol peroxidase (POX), (Figure 4A) is lower in 50 Gy (6.04±1.99)and 150 Gy (17.53±4.58) and high in case of 100Gy (27.20±0.45), 200 Gy (31.68± 1.4) as compared to non-irradiated control indicating the rapid dehydrogenation of H202 in presence of substrate guaiacol and plays an important role in detoxification of H2O2 to H2O (p ˂0.05). In contrast, activity of catalase (CAT) effectively higher in 50 Gy (0.46±0.03) but continually decreased towards 200 Gy (0.13±0.020, p<0.05).This indicates activation of POX, that plays an important role in detoxification mechanism and accumulates flavonoids (r=0.999, p<0.01), phenolics (r=0.997, p<0.01) and carotenoids (r=0.998, p<0.01) (Figure 4B). Activity of superoxide dismutase (SOD) effectively increases from 50 Gy (28.7±0.60) to 200 Gy (49.2±0.88) shows rapid dismutation of O2 – to H2O2 and molecular oxygen (Figure 4C) and accumulates high non-enzymatic antioxidants (0.978,0.988,0.971 correlation at p< 0.01).
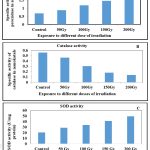 |
Figure 4: A: represents variation in peroxidase activity; B: Catalse activity in different doses; C: shows variation in SOD activity. Means with variations are significantly different at P˂ 0.05 level of significance |
Estimation of photosynthetic parameters
Total chlorophyll was determined from third leaves of plants of different doses and expressed in terms of mg per gm of FW (milligram per gram fresh weight). Chlorophyll a content increased from 50 Gy (22.3±1.15,p< 0.05) to higher doses (100Gy,33.58±1.10) doses as compare to control ( p ˂0.05; Figure 5A). But there was decrement in chlorophyll a content from 150 Gy (18.48±0.49) and 200 Gy (16.68±0.66) may be due to effect of lipid peroxidation in 150 Gy (0.19±0.010), 200Gy (0.28±0.015) in thylakoid membrane (correlation at r = -0.568) A similar pattern was observed for chlorophyll b and total chlorophyll concentration and the variation was significant according ANNOVA and POST HOC analysis. Concentration of chlorophyll at different doses is shown in Figure 5a. Total soluble sugar was expressed in terms of µg per gram of FW and no significant change was observed in concentration of total soluble sugar in different doses as compared to control (Figure 5B) indicating gamma irradiation did not affect photosynthetic end products due to activated antioxidative mechanism (p< 0.05, r= 0.749,0.758) even though chlorophyll content decreased (correlation at r = -0.723, Fig 5b).
 |
Figure 5: A: Effect of irradiation in content of chlorophyll a, chlorophyll b and total chlorophyll for different doses; B: Concentration of total soluble sugar from leaves from different dose. Means with variation significantly different at P˂ 0.05 level of significance. |
Discussion
There are many earlier reports available on the effect of radiation on the biochemical and physiological changes in crop plants. In the present investigation, gamma irradiated seeds (50 Gy, 100 Gy,150 Gy,200 Gy) compared with plants without any treatments and act as reference plants .The third leaf collected from each irradiated dose was used for biochemical and physiological characterization.
When plants are exposed to ionizing radiation, they produced ROS such as H2O2, O2 – , and OH– due to disruption of cellular homeostasis 34 and in response to these adverse condition plants produce enzymatic and non-enzymatic antioxidants35 to detoxify ROS. An important compound flavonoids complexes with chelating ions, iron or copper has capacity to prevent generation of ROS36 .In the present investigation, it was shown that effective dose (50 Gy, 100Gy) of irradiation accumulates a high concentration of phenolics and flavonoids which were also observed from the study of effect of gamma radiation on phenolic compounds in rice.37,38 Carotenoids have a strong antioxidant capacity to scavenging peroxy-acyl free radicals more efficiently because of their conjugated double bonds.38 Concentration of carotenoids, flavonoids and phenolics also increased to scavenge ROS during stress causing oxidative stress shown a positive correlation with total proline , an important stress indicator( correlation at r =0.906,0.967,0.886,p< 0.05,0.01).39 Some of the parameters commonly used as oxidative stress biomarkers, are levels of H2O2 and O2– radical accumulation, lipid peroxidation.40,41 The current study reveals, at higher doses (200Gy) concentration of H2O2 is higher as compare O2 – (correlation statistics r= -0.718). This is because of enzyme superoxide dismutase (SOD) dismutase O2 – to H2O2 and molecular oxygen and acts as first line of defence against ROS generation 42. Peroxidation of lipid membranes is a reflection of stress induced damage at cellular level.43 Further confirmation was done by measuring MDA content that shown to be increased from lower to higher radiation doses indicating the level of cellular damage due to rapid peroxidation, also reported by.44 Accumulation of proline as a stress indicator is also effectively higher with respect to high doses and acts as an important cellular osmoticum against exposure to high doses of irradiation.45
In the present context, activity of peroxidase was increased from in higher doses (p< 0.05) but reverse was happened in catalase show negative correlation at r = -0.925, p< 0.01).Thus, peroxidase might take important role in detoxification process by activating non-enzymatic antioxidative mechanism, shown positive correlation for flavonoids, phenols and carotenoids(p< 0.05). Significant change was observed in superoxide dismutase for rapid dismutation of O2 – to H2O2 and to accumulation of antioxidants at high concentration (significant at p< 0.05), also enhancing accumulation of high amount of proline as cellular osmolytes (significant at p<0.01). Earlier studies also reported by46 that, the concentration of peroxidase (POX) and Super oxide dismutase (SOD) increases when exposes to gamma radiation. How the irradiation affects photosynthetic precursors was measured by estimation of total chlorophyll content. Total chlorophyll content was increased in upto effective dose (100 Gy) may be because of accumulation of O2– during water hydrolysis.20,47 Accumulation of total chlorophyll decreased when exposed to high doses (200Gy) , may be disturbances in its biosynthetic precursor which was also observed by.48 Concentration of chlorophyll a was higher as compare to chlorophyll b, may be because of disturbances in its metabolic pathways during biosynthesis.49 Hormesis is a biphasic phenomenon of excitation or stimulatory effect of any agent by a small dose in any system and has modulatory effect on plants, whereas higher doses have inhibitory effect on system of plants.50 Thus, from the above investigation, it was studied that, effective dose (50Gy,100 Gy) of radiation stimulating the biochemical and physiological changes in a positive way, thus future analysis in this perspective may give quality traits for future breeding programmes.
Table 1: Simple correlation coefficients (r) between different irradiation doses from 50Gy – 200Gy.
| TF | TP | TC | THP | TSO | TPO | TCA | TSOD | LP | TPR | TChla | TChlb | TChl a+b | TSS | |
| TF | 1 | |||||||||||||
| TP | .981** | 1 | ||||||||||||
| TC | .999** | .970** | 1 | |||||||||||
| THP | .639 | .634 | .630 | 1 | ||||||||||
| TSO | -.130 | -.071 | -.134 | -.718 | 1 | |||||||||
| TPO | .999** | .977** | .998** | .647 | -.121 | 1 | ||||||||
| TCA | -.931* | -.982** | -.913* | -.554 | -.051 | -.925* | 1 | |||||||
| TSOD | .978** | .988** | .971** | .531 | .058 | .976** | -.974** | 1 | ||||||
| LP | .647 | .585 | .651 | .752 | -.784 | .627 | -.466 | .493 | 1 | |||||
| TPR | .906* | .967** | .886* | .514 | .120 | .901* | -.997** | .964** | .392 | 1 | ||||
| TChla | -.193 | -.070 | -.220 | .095 | .304 | -.163 | -.040 | -.075 | -.568 | .104 | 1 | |||
| TChlb | .118 | .233 | .092 | .287 | .281 | .150 | -.325 | .230 | -.384 | .383 | .951* | 1 | ||
| TChla+b | .317 | .439 | .287 | .396 | .247 | .342 | -.532 | .429 | -.223 | .582 | .865 | .972** | 1 | |
| TSS | .749 | .695 | .758 | .162 | -.032 | .722 | -.640 | .716 | .625 | .599 | -.723 | -.499 | -.299 | 1 |
Conclusion
Biochemical and physiological characterization are widely used in the development of good quality traits and high-performance varieties that are useful for farmer cultural practices. In the present study, black rice seeds were exposed to gamma irradiation (50 Gy, 100 Gy, 150 Gy, 200 Gy) and compared with control i. e without irradiation. Physiological and biochemical characteristics were measured to know the effect of irradiation that may helped to know any metabolic changes in respective crop. Exposure to physical mutagen accumulates more amount of antioxidants to avoid harmful effect of free radicals on cellular metabolites. Plants releases antioxidative compounds in response to avoid damage through reactive oxygen species (ROS) including superoxide radicals (O2-.), hydrogen peroxide (H2O2). Catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD) are some of the important enzymes that plays a principal role in scavenging these free radicals. In conclusion, effective irradiation doses might be useful to screening of qualitative traits that may helped to further analysis in molecular dimensions of selected doses and also explore their role in future plant breeding programs in black rice.
Acknowledgment
The authors are highly obliged to Head of School of Biotechnology, Gangadhar Meher University for providing all the necessary facilities for the experiment. The authors are also thankful to The Indian Institute of Technology, Kharagpur especially Agricultural and Food Engineering Department for providing necessary laboratory facilities for completion of the current study.
Conflict of Interest
Authors have no conflict of interest
Funding Sources
There is no funding sources.
References
- Singh Y, Singh US. Genetic diversity analysis in aromatic rice germplasm using agro-morphological traits. Indian Journal of plant Genetic resources.2008: 21(1):37-42.
- Manjunatha B, Kusagur N, Kumara BN.Variability, Heritability and Genetic Advance Studies in Advanced Genotypes of Rice (Oryza sativa L.).2020: Int. J. Curr. Microbiol. App. Sci. 9(8):1668-70.
CrossRef
- Sarla N, Swamy BM ;Oryza glaberrima: a source for the improvement of Oryza sativa. Current science. 2005: Sep 25:955-63.
- Kumar M, Kumar RR Genetic Variability Parameters for Yield and Yield Related Traits in Rice (Oryza sativa L.) under Irrigated and Drought Stress Condition. Int. J. Curr. Microbiol. App. Sci. 2020:9(2):1137-43.
CrossRef
- Agrawal A. Black Rice the New black gold of India. Food and Agriculture Spectrum Journal. 2021 30;2(03):237-40.
- Lila MA. Anthocyanins and human health: an in vitro investigative approach. Journal of Biomedicine and Biotechnology. 2004 :12;2004(5):306.
CrossRef - Pedro AC, Granato D, Rosso ND: Extraction of anthocyanins and polyphenols from black rice (Oryza sativa L.) by modeling and assessing their reversibility and stability. Food Chemistry. 2016 Jan 15;191:12-20.
CrossRef - Saxena R.K., T.T. Chang, R.L. Sapra and R.S. Paroda 1088. Evaluation studies inindigenous rice (Oryza sativa L.) germaplasm at IRR1. Philippines. Published in NBPGR manual pp. 1-3
- Nayak AR, Reddy JN, Pattnaik AK.Quality evaluation of some Thailand and Vietnam scented rice. Indian Journal of Plant Genetic Resources. 2002:15(2):125-7.
- Golam F, Yin YH, Masitah A, Afnierna N, Majid NA, Khalid N, Osman M Analysis of aroma and yield components of aromatic rice in Malaysian tropical environment. Australian Journal of Crop Science. 2011:1;5(11):1318-24.
- Ichikawa, H., Ichiyanagi, T., Xu, B., Yoshii, Y., Nakajima, M. and Konishi.T.Antioxidant activity of anthocyanin extract from purple black rice. Journal of medicinal food, 2001:4(4), pp.211-218.
CrossRef
- Hamid, A.A., Aiyelaagbe, O.O., Usman, L.A., Ameen, O.M. and Lawal, A Antioxidants: Its medicinal and pharmacological applications. African Journal of pure and applied chemistry, 2010:4(8), pp.142-151
CrossRef
- Khah MA, RC Verma..Assessment of the effects of gamma radiations on various morphological and agronomic traits of common wheat (Triticum aestivum L.) var. WH-147. European Journal of Experimental Biology.2015:5 (7):6-11.
CrossRef
- Kim JH, MH Baek , BY Chung , SG Wi , JS Kim. Alterations in the photosynthetic pigments and antioxidant machineries of red pepper (Capsicum annuum L.) seedlings from gamma-irradiated seeds. Journal of Plant Biology. 2004: 47(4):314-21. https://doi.org/10.1007/BF03030546
CrossRef - Kovacs, E., & Keresztes. A. Effect of gamma and UV-B/C radiation on plant cells. Micron 2002:3(2), 199-210. https://doi.org/10.1016/S0968-4328(01)00012-9
CrossRef - Sirisoontaralak P, Noomhorm A. Changes to physicochemical properties and aroma of irradiated rice. Journal of stored products research. 2006:Jan 1;42(3):264-76.
CrossRef
- Kant, A. and Chakraborty, N.R., (2021); Induction of mutation through gamma irradiation in non-basmati aromatic ‘badshabhog’rice (Oryza sativa L.).
CrossRef - Bhat, N.A., Wani, I.A. and Sultan, N.Effect of Gamma-irradiation on the Physicochemical, Functional, and Antioxidant Properties of Unpigmented BrownWhole Rice Flour. Food Science and Technology International, 2022:p.10820132211069244
CrossRef
- Gaikwad DD, AD Chapolikar, CG Devkate, KD Warad, AP Tayade, RP Pawar, AJ Synthesis of indazole motifs and their medicinal importance: An overview. European journal of medicinal chemistry.2015:90:707-31. https://doi.org/10.1016/j.ejmech.2014.11.029
CrossRef - Kumar M, A Basu , P Kumari , S Jha , A Mitra .Tobacco plantlets ameliorate oxidative stress upon expression of a cryptogein gene. Plant Cell, Tissue and Organ Culture (PCTOC). 2016: 125(3):553-70.. DOI 10.1007/s11240-016-0970-0
CrossRef - Chang CC, MH Yang , HM Wen , JC Chern. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of food and drug analysis 2002:10(3):3. https://doi.org/10.38212/2224-6614.2748
CrossRef - Tang Y, W Cai , B Xu . Profiles of phenolics, carotenoids and antioxidative capacities of thermal processed white, yellow, orange and purple sweet potatoes grown in Guilin, China. Food Science and Human Wellness.20151;4(3):123-32. https://doi.org/10.1016/j.fshw.2015.07.003
CrossRef - Moradi F, AM Ismail .Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Annals of botany. 2007:1;99 (6):1161-73. https://doi.org/10.1093/aob/mcm052
CrossRef - Bates LS, RP Waldren , ID Teare (1973).Rapid determination of free proline for water-stress studies. Plant and soil. 1973: 39(1):205-7. https://doi.org/10.1007/BF00018060
CrossRef - Qiu X, C Lei , L Huang , X Li , H Hao , Z Du , H Wang , H Ye. L Beerhues , B Liu . Endogenous hydrogen peroxide is a key factor in the yeast extract-induced activation of biphenyl biosynthesis in cell cultures of Sorbus aucuparia. Planta.2012:235(1):217-23. https://doi.org/10.1007/s00425-011-1545-2
CrossRef - He L, Z Gao Pretreatment of seed with H2O2 enhances drought tolerance of wheat (Triticum aestivum L.) seedlings. African Journal of Biotechnology.2009:8(22). 5897/AJB09.490
CrossRef - Ni Z, Kim ED, Ha M, Lackey E, Liu J, Zhang Y, Sun Q, Chen ZJ. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature.2009: 457(7227):327-31. https://doi.org/10.1038/nature07523
CrossRef - Fayez KA, Bazaid SA. Improving drought and salinity tolerance in barley by application of salicylic acid and potassium nitrate. Journal of the Saudi Society of Agricultural Sciences 2014:1;13(1):45-55. https://doi.org/10.1016/j.jssas.2013.01.001
CrossRef - Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry.1976 :72(1-2):248-54.
CrossRef - Chance B, Maehly AE. Methods in Enzymol. by SP Colowick and NO Kaplan, Academic Press, Inc., New York.1957:4:273.
- Lee JH.Apr. Identification and quantification of anthocyanins from the grains of black rice (Oryza sativa L.) varieties. Food Science and Biotechnology.2010:19(2):391-7. https://doi.org/10.1007/s10068-010-0055-5
CrossRef - Mukherjee C, Sircar D, Chatterjee M, Das S, Mitra A. Combating photooxidative stress in green hairy roots of Daucus carota cultivated under light irradiation. Journal of plant physiology.2014: 171(2):179-87. https://doi.org/10.1016/j.jplph.2013.10.013
CrossRef - Nikravesh F, Khavari-Nejad RA, Rahimian H, Fahimi H. Mar. Study of antioxidant enzymes activity and isozymes pattern in hairy roots and regenerated plants in Nicotiana tabacum. Acta physiologiae plantarum. 2012:34(2):419-27.
CrossRef - Sharma P, Jha AB, Dubey RS, Pessarakli M . Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. 2012. Journal of botany.;2012.
CrossRef - Noctor G, Foyer CH.Ascorbate and glutathione: keeping active oxygen under control. 1998.Annual review of plant biology. 1998 Jun;49(1):249-79.
CrossRef - Zhu F, Cai YZ, Bao J, Corke H. Effect of γ-irradiation on phenolic compounds in rice grain. Food chemistry. 2010:1;120(1):74-7. https://doi.org/10.1016/j.foodchem.2009.09.072
CrossRef - Archanachai, K., Teepoo, S. and Sansenya, S Effect of gamma irradiation on growth, proline content, bioactive compound changes, and biological activity of 5 popular Thai rice cultivars. Archanachai, K., Teepoo, S. and Sansenya, S., 2021. Journal of bioscience and bioengineering,2021:132(4), pp.372-380.
CrossRef - Nimse, S.B. and Pal, D.Free radicals, natural antioxidants, and their reaction mechanisms. RSC advances, 2015:5(35), pp.27986-28006.
CrossRef - Al Hassan, M., FUERTES, M.M., SÁNCHEZ, F.J.R., Vicente, O. and Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. 2015.Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 2015:43(1), pp.1-1.
CrossRef - Hakmaoui A, Pérez-Bueno ML, García-Fontana B, Camejo D, Jiménez A, Sevilla F, Barón M.Analysis of the antioxidant response of Nicotiana benthamiana to infection with two strains of Pepper mild mottle virus. Journal of experimental botany.2012: 63(15):5487-96. https://doi.org/10.1093/jxb/ers212
CrossRef - Chandrashekar, K.R., Somashekarappa, H.M. and Souframanien.J.Effect of gamma irradiation on germination, growth, and biochemical parameters of Terminalia arjuna Roxb. 2013.Radiation Protection and Environment, 36(1), p.38.
CrossRef - Bowler, C., Montagu, M.V. and Inze, D.Superoxide dismutase and stress tolerance. .Annual review of plant biology, 1992:43(1), pp.83-116.
CrossRef - Alscher, R.G., Erturk, N. and Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. Journal of experimental botany, 2002:53(372), pp.1331-1341.
CrossRef - Jain M, Mathur G, Koul S, Sarin N. .Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Reports. 2001:20(5):463-8. https://doi.org/10.1007/s002990100353
CrossRef - Choi, H.I., Han, S.M., Jo, Y.D., Hong, M.J., Kim, S.H. and Kim, J.B. Effects of acute and chronic gamma irradiation on the cell biology and physiology of rice plants. 2021. Plants, 2021:10(3), p.439.
CrossRef - Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019).The roles of environmental factors in regulation of oxidative stress in plant. 2019 May 8 BioMed research international.;2019.
CrossRef - Moussa, H (2011).Low dose of gamma irradiation enhanced drought tolerance in soybean. Acta Agronomica Hungarica, 2011:59(1), pp.1-12.
CrossRef - Marcu D, Cristea V, Daraban L . .Dose-dependent effects of gamma radiation on lettuce (Lactuca sativa var. capitata) seedlings. International Journal of Radiation Biology.2013:89 (3):219-23. https://doi.org/10.3109/09553002.2013.734946
CrossRef - Borzouei, A., Kafi, M., Khazaei, H., Naseriyan, B. and Majdabadi, A.Effects of gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings.2010. J. Bot, 42(4), pp.2281-2290.
- Calabrese, E.J.Overcompensation stimulation: a mechanism for hormetic effects.Critical Reviews in Toxicology, 2001:31(4-5), pp.425-470
CrossRef

