Introduction
Plants are a major source of food, fiber, fodder, medicines, and many other valuable products for humans. Individual parts of plants such as roots stem, leaves, fruits, flowers, and seeds are used for people’s daily needs. A variety of insects, bacteria, viruses, fungi, and other pests invade plants at various stages of development, reducing productivity and causing great loss to humankind. More than 800 million people in developing countries lack adequate food supplies, with plant diseases causing at least 10% of their food loss.
Plant Description
Cumin seeds are mainly used as a spice in the form of essential ingredients in all mixed spices and curry powders for flavoring vegetables, pickles, soups, sausages, cheeses, and other dishes, bread, cakes, and biscuits. The chemical composition includes 2.5- 4.5% volatile oils, 10% fixed oils, and proteins. Volatile oils are mainly composed of 30- 50% cumin aldehyde, minor amounts of b-pinene, phellandrene, cumin alcohol, hydrated aldehydes, and hydrocumin, which are suitable for medicinal purposes. The aromatic oil of cumin seeds is also used as a flavoring in curries, liqueurs, and liqueurs and has excellent uses in the perfume industry. The residue left after precious oil extraction contains 17.2% protein and Fat. It is a seasonal crop grown during the Rabi period. It is also grown as a cover crop under rain-fed and irrigated conditions after caliph crops such as jowar, green gram, cowpea, and maize. Cumin is mainly grown in the two states of India, namely Gujarat, and Rajasthan.2
Scenario and status of cumin production in India
India is the world’s leading producer (70% of global production), consumer, and exporter of cumin. Almost 80% of crops are grown in India.3 In 2021-2022, the cumin planted area was 151,849 hectares, with production and productivity of 81,700 tons and 6,700 tons/ha respectively. Cumin is only grown in Gujarat and Rajasthan. Gujarat was the leading state for cumin production in India from 2010 to 2019. Gujarat had the highest productivity in 2018 where he was 1.04 MT/ha 4 West Bengal, Punjab, Uttar Pradesh, and Andhra Pradesh contribute to India’s production. Gujarat’s core cumin-producing districts are Surendranagar, Banaskantha, Jamnagar, and Patan. Bama, Jalore, Jodhpur, and Nagpur in Rajasthan are major cumin-producing areas.5
Cumin diseases
Cumin is an important rabbi crop in parts of Saurashtra and parts of northern Gujarat and is mainly affected by three major diseases: blight (Alternaria burnsii) and wilt (Fusarium oxysporum f. Sp. cumini), and powdery mildew (Erysiphe polygoni). Cumin is an important rabi crop in the part of Saurashtra and North Gujarat and is affected mainly by three important diseases viz., blight (Alternaria burnsii), wilt (Fusarium oxysporum f. Sp. cumini), and powdery mildew (Erysiphe polygoni). Wilt is the most destructive disease of cumin plants. Gaur (1949) first reported the existence of wilt disease caused by Fusarium species in Rajasthan, India. Plants are affected at all stages of growth, but the severity of wilting increases with plant age. When the plant height reaches 2.5cm to 5.0cm, it will die. In older plants, the leaf color changes from green to yellow, starting with the oldest leaves and moving to the younger leaves. In severe stages, the tops and leaves of the plant fall off, leading to complete plant death. Such plants are easy to pull out of the ground. The roots of diseased plants have dark brown spots. Sometimes only partial wilting is observed. If plants become infected during flowering, they remain sterile.6 The present study was conducted to evaluate the antifungal activity of selected plants against the growth of Fusarium oxysporum f.sp. Cumini causes cumin wilting.
Materials and Methods
Collection of native plants in and around Rajkot District
Seven plant species were used in this study to evaluate antifungal activity (Table 1). Native plants were selected based on their antibacterial properties and abundant arable land. Nominated plants are well adapted to climatic conditions and popular with local residents for their medicinal properties.7 Selected plant leaves/flowers were collected from different villages in the Rajkot district.
Fungal strains
Pure culture of Fusarium oxysporum f. sp. cumini was acquired from the Plant pathology department of Agriculture University, Junagadh (Figure1).
Preparation of aqueous extract
Fresh Leaves/flowers of each plant were washed theory with tap water followed by sterilized distilled water. 100gm of these Leaves/ flowers were crushed in 100 ml of distilled water in a grinder. The extracts were strained through a muslin cloth to remove plant debris. The filtrate thus obtained was designated as stock solution. 5%, 10%, and 15% plant extract were prepared from the stock solution of each plant.8 Similarly, each plant extract was prepared in acetone (95%) and cow urine(untreated).
Antifungal activity assay of botanical extracts by using poison food technique
1 ml of each plant extract at different concentrations of 5%, 10%, and 15% was added to 15 ml of sterile molten potato dextrose agar in a sterile Petri dish. Fusarium oxysporum has grown at 7 days of age. A 5 mm diameter mycelial disc of the culture was placed in the center of the petri dish containing the extract of each plant. A plate without plant extract served as a negative control. Plates were incubated at 27±2°C. Triplicates were maintained for each treatment in this assay. Radial mycelial growth was measured after 6 days of incubation (Figure 2). Results were compared to negative controls.9
Statistical Analysis
Experiments were repeated three times and the average of the three measurements was used for calculations. Percentage inhibition was determined using the average colony diameter and calculated using the following formula proposed by Vincent (1947).
L = [(C – T)/C] x 100
where L is the inhibition rate, C is the colony radius of the control plate, and T is the radial growth of Fusarium oxysporum in the presence of plant extracts.10
Statistical analysis of results was performed using R language version 4.1.1.1. One-way analysis of variance (ANOVA) at values of p ≤ 0.001, followed by Tukey’s post hoc test at p ≤ 0.05, was used to determine significant differences between results obtained in each experiment.
Result
Extracts from seven different plants were tested for antifungal activity at concentrations of 5%, 10%, and 15%. Radial growth of Fusarium oxysporum f.sp. is measured, and the percent inhibition is calculated for each plant. (Tables 2, 3, and 4).
Antifungal Activity Test of Aquatic Plant Extracts Using the Poison Food Method.
The highest inhibition of mycelial growth was recorded with 15% aqueous extract from Senna alexandrina (84.54%), followed by Azadirachta indica (73.35%). At the same concentrations, extracts of Psidium guajava, Asparagus racemosus, TriumfettaPilosa, and Ocimumtenuiflorum inhibited mycelial growth in the range of 68.01% to 60.45%, while Aloe barbadensis miller showed minimal inhibition. (39.22%).
At a concentration of 10% in water, the most effective extracts to inhibit mycelial growth were Senna alexandrina (78.05%), followed by Psidium guajava (61.47%). Other extracts tested showed potency from 58.00 % to 46.64 % against Azadirachta indica, Asparagus racemosus, Triumfetta Pilosa and Ocimumtenuiflorum. Aloe barbadensis miller extract showed the lowest potency (34.94%).
At a concentration of 5% in water, the most effective extract to inhibit mycelial growth was Senna alexandrina (75.28%) followed by Psidium guajava (55.43%). Other tested extracts such as Azadirachta indica, TriumfettaPilosa, Asparagus racemosus, Senna alexandrina and Ocimumtenuiflorum showed potency between 53.78% and 36.54%. The least effective was Aloe barbadensis miller 59.57% (Figure 3).
Antifungal Activity Test of Plant Extracts in Acetone Using Poison Food Method
For plant extracts prepared with acetone, the highest inhibition of mycelial growth was recorded by Azadirachtaindic (89.88%) at a concentration of 15%, followed by Senna alexandrina (88.56%). Other tested extracts showed potencies of 78.38% to 64.15% against Psidium guajava, Asparagus racemosus, Triumfettapilosa and Ocimumtenuiflorum. The lowest efficacy was recorded by Aloe barbadensis miller (58.17 %).
At 10% concentration in acetone, Senna alexandrina (84.80%) was the most effective extract to inhibit mycelial growth, followed by Triumfetta Pilosa (82.71%). Other extracts tested showed potencies between 81.94 % and 62.76 % for Azadirachta indica, Psidium guajava, Asparagus racemosus and Ocimumtenuiflorum. The least effective was Aloe barbadensis miller (56.18%).
At a concentration of 5% in acetone, the most effective extract to inhibit mycelial growth was observed from Senna alexandrina (83.84%), followed by Triumfettapilosa (77.93%). Other tested extracts such as Azadirachtaindic, Psidium guajava, Asparagus racemosus and Ocimumtenuiflorum
showed potency from 76.36% to 56.85%. The least effective was Aloe barbadensis miller (46.08%) Figure:4.
Antifungal Activity Test of Plant Extracts in Cow Urine Using Poison Food Method.
In plant extracts prepared from cow urine, Triumfettapilosa recorded the highest inhibition (84.33%) at a concentration of 15%, followed by Psidium guajava (82.0 %). At the same concentrations, extracts of Azadirachta indica, Asparagus racemosus, Senna alexandrina and Ocimumtenuiflorum showed potencies between 74.95 % and 67.77%. The least effective was Aloe barbadensis miller (30.48%).
At concentrations of 10% with cow urine, the most effective extracts to inhibit mycelial growth were Triumfetta Pilosa (77.91%) and Psidium guajava (77.34%). Other extracts tested included Azadirachta indica, Asparagus racemosus, Senna alexandrina and Ocimumtenuiflorum showed efficiency ranging from 69.57% to 61.44%. The lowest effect was recorded by Aloe barbadensis miller (48.03 %).
At 5% concentration of cow urine, the most effective extract to inhibit mycelial growth was Psidium guajava (73.74%) followed by Triumfetta pilosa (72.32%). Other tested extracts such as Azadirachta indica, Asparagus racemosus, Senna alexandrina and Ocimumtenuiflorum showed potencies between 66.05% and 59.57%. A decrease in potency was recorded by Aloe barbadensis miller (43.88%).
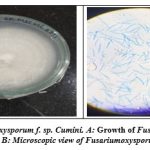 |
Figure 1: Fusarium oxysporum f. sp. cumini |
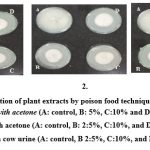 |
Figure 2: Evaluation of plant extracts by poison food technique on PDA media. |
Table 1: List of Plant and parts used for evaluation
| Sr.No. | Common name | Scientific name | Family | Plant part used |
| 1 | Neem | Azadirachta indica | Mahogany | Leaves |
| 2 | Guava | Psidium guajava | Myrtaceae or myrtle | Leaves |
| 3 | Shatavari | Asparagus racemosus | Asparagaceae | Leaves |
| 4 | Aelovera | Aloe barbadensis miller | Asphodelaceae | Leaves |
| 5 | Jipto | Triumfettapilosa | Malvaceae | Leaves |
| 6 | Aavedi | Senna alexandrina | Febaceae | Flower |
| 7 | Tilak tulsi | Ocimumtenuiflorum | Lamiaceae | Leaves |
Table 2: In vitro evaluation of plant extracts in the water against Fusarium oxysporum f.sp. cumini.
|
Treatment |
Colony Diameter(mm) *at
conc |
Average Colony Diamete r
(mm) |
% Inhibition*
±Standard Deviation |
Average % Inhibition | ||||
| 5% | 10% | 15% | 5% | 10% | 15% | |||
| Azadirachta indica | 50.95 ±0.65 |
38.84 ±1.3 |
24.98 ±0.85 |
38.25 ±0.95 |
44.5 ±0.72 |
57.94 ±1.52 |
73.35 ±0.9 |
58.60 ±1.06 |
| Psidium guajava | 40.11 ±0.16 |
34.67 ±0.58 |
28.78 ±1.20 |
34.52 ±0.64 |
55.43 ±0.18 |
61.47 ±0.64 |
68.01 ±1.34 |
61.64 ±0.72 |
| Asparagus racemosus | 58.11 ±0.26 |
49.01
±0.07 |
34.99
±0.50 |
47.37 ±0.27 |
36.54 ±0.28 |
46.64
±0.08 |
62.22 ±0.55 |
48.74 ±0.30 |
| Aloe barbadensis miller | 65.92 ±0.30 |
65.5 ±0.5 |
62.69 ±0.14 |
64.05 ±0.32 |
32.30 ±0.34 |
34.94 ±0.6 |
39.22 ±0.1 |
35.49 ±036 |
| TriumfettaPilosa | 41.59 ±0.65 |
37.9 ±0.13 |
35.59 ±0.9 |
38.36 ±0.56 |
53.78 ±0.7 |
57.88 ±0.1 |
60.45 ±1.0 |
57.37 ±0.63 |
| Senna alexandrina | 22.24 ±0.71 |
19.74 ±0.83 |
13.91 ±0.60 |
18.63 ±0.71 |
75.28 ±0.79 |
78.05 ±0.92 |
84.54 ±0.6 |
79.29
±.79 |
| Ocimumtenuiflorum | 42.65 ±0.95 |
37.79 ±0.61 |
32.85 ±0.9 |
37.76 ±0.82 |
52.60 ±1.06 |
58.00 ±0.68 |
63.5 ±1.00 |
58.03 ±0.91 |
| Control | 90.00 ±0.00 |
90.00 ±0.00 |
90.00 ±0.00 |
90.00 ±0.00 |
0.00 | 0.00 | 0.00 | 0.00 |
*Mean of three replications, Significant at p≤0.01 level according to Tukey’s Post Hoc test
Table 3: In vitro evaluation of plant extracts in Acetone against mycelial growth of Fusarium oxysporum f. sp. cumini
| Plant extracts | Colony Diameter (mm) *at conc. | Average Colony Diameter (mm) | % Inhibition*
±Standard Deviation |
Average % Inhibitio n |
||||
| 5% | 10% | 15% | 5% | 10% | 15% | |||
| Azadirachtaindic | 21.27 ±0.72 |
16.25 ±0.39 |
9.11 ±0.37 |
15.54 ±0.49 |
76.36 ±0.80 |
81.94 ±0.43 |
89.87 ±0.42 |
82.72 ±0.55 |
| Psidium guajava | 31.8 ±0.60 |
22.35 ±0.58 |
19.45 ±0.48 |
24.53 ±0.55 |
64.66 ±0.66 |
75.16 ±0.65 |
78.38 ±0.53 |
72.73 ±0.61 |
| Asparagus racemosus | 38.40 ±0.63 |
33.51 ±0.52 |
32.26 ±0.34 |
34.72 ±0.49 |
57.32 ±0.70 |
62.76 ±0.57 |
64.15 ±0.38 |
61.41 ±0.55 |
| Aloe barbadensis miller | 50.52 ±0.81 |
41.43 ±0.72 |
39.64 ±1.38 |
43.86 ±0.97 |
46.08 ±0.90 |
56.18 ±0.80 |
58.17 ±1.54 |
53.47 ±1.08 |
| Triumfettapilosa | 19.85 ±0.89 |
15.55 ±1.11 |
8.66 ±0.64 |
14.68 ±0.88 |
77.93 ±0.99 |
82.71 ±1.23 |
90.37 ±0.71 |
83.67 ±0.97 |
| Senna alexandrina | 15.08 ±0.81 |
13.67 ±0.37 |
10.29 ±0.84 |
13.01 ±0.67 |
83.24 ±0.90 |
84.80 ±0.41 |
88.56 ±0.94 |
85.53 ±0.75 |
| Ocimumtenuiflorum | 70.83 ±0.5 |
60.16 ±0.0 |
59.10 ±0.47 |
63.36 ±0.36 |
56.85 ±0.62 |
68.70 ±0.07 |
69.88 ±0.52 |
65.14 ±0.40 |
| Control | 90.00 ±0.00 |
90.00 ±0.00 |
90.00 ±0.00 |
90.00 ±0.00 |
0.00 | 0.00 | 0.00 | 0.00 |
*Mean of three replications, Significant at p≤0.01 level according to Tukey’s Post Hoc test
Table 4: In vitro evaluation of plant extracts in Cow urine against mycelial growth of Fusarium oxysporum f. sp. Cumini
|
Treatment |
Colony Diameter(mm) *at conc
Mean±SD |
Average Colony Diamete r
(mm) |
% Inhibition*
±Standard Deviation |
Average % Inhibiti-on | ||||
| 5% | 10% | 15% | 5% | 10% | 15% | |||
| Azadirachta indica | 36.51 ±0.59 |
34.81 ±0.83 |
32.25 ±0.92 |
34.52 ±0.63 |
61.64 ±0.65 |
63.53 ±0.42 |
66.38
±1.02 |
63.85 ±0.69 |
| Psidium guajava | 23.62 ±0.76 |
20.38 ±0.67 |
16.19 ±0.34 |
20.06 ±0.59 |
73.74 ±0.85 |
77.34 ±0.74 |
82.00 ±0.37 |
77.70 ±0.65 |
| Asparagus racemosus | 39.88 ±0.46 |
36.70 ±0.55 |
31 ±0.51 |
35.86 ±0.50 |
57.90 ±0.51 |
61.44 ±0.61 |
67.77 ±0.56 |
62.37 ±0.56 |
| Aloe barbadensis miller | 54.50 ±0.26 |
52.56 ±0.71 |
58.56 ±0.24 |
55.20 ±0.40 |
43.88 ±0.29 |
48.03 ±0.78 |
30.48 ±0.26 |
40.80 ±0.44 |
| Triumfettapilosa | 24.90 ±0.37 |
18.82 ±1.03 |
14.1 ±0.27 |
19.27 ±0.55 |
72.32 ±0.41 |
77.91 ±0.86 |
84.33 ±0.30 |
78.19 ±0.52 |
| Senna alexandrina | 30.54 ±0.28 |
27.38 ±0.79 |
22.53 ±0.4 |
26.81 ±0.50 |
66.05 ±0.31 |
69.57 ±0.88 |
74.95 ±0.50 |
70.19 ±0.56 |
| Ocimumtenuiflorum | 36.38 ±0.84 |
34.16 ±1.11 |
24.81 ±0.75 |
31.37 ±0.9 |
59.57 ±0.37 |
62.03 ±1.23 |
72.42 ±0.83 |
64.68 ±0.82 |
| Control | 90.00 ±0.00 |
90.00 ±0.00 |
90.00 ±0.00 |
90.00 ±0.0 |
0.00 | 0.00 | 0.00 | 0.00 |
*Mean of three replications, Significant at p≤0.01 level according to Tukey’s Post Hoc test
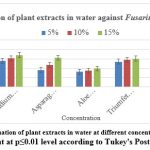 |
Figure 3: In vitro evaluation of plant extracts in water at different concentration on PDA media |
Significant at p≤0.01 level according to Tukey’s Post Hoc test
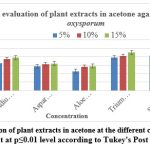 |
Figure 4: In vitro evaluation of plant extracts in acetone at the different concentration on PDA media. |
Significant at p≤0.01 level according to Tukey’s Post Hoc test
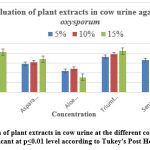 |
Figure 5: In vitro evaluation of plant extracts in cow urine at the different concentrations on PDA media |
Significant at p≤0.01 level according to Tukey’s Post Hoc test
Discussion
The antifungal activity of plant extracts may be due to the presence of some antifungal toxins. The presence of antifungal compounds in higher plants is well-known and valuable in controlling plant diseases.11 Management of Fusarium wilt in cumin is primarily based on the application of chemical fungicides, the use of pathogen-resistant cultivars, and crop rotation. The application of chemical fungicides has been the traditional method of controlling wilt disease caused by Fusarium oxysporum. Cumin is a major health hazard for which new eco-friendly fungicides need to be found.12 The results of this study showed that the plant extracts of Azadirachta indica, Psidium guajava, Senna alexandrina, and Triumfetta pilosa were most effective against Fusarium oxysporum causes cumin wilt. These results correlate with previous studies on the antifungal activity of different plant extracts, namely Azardiachta indicia, Artemessia Annua, Ocimum sanctum, and Rheum emodi, against the pathogen Fusarium solani at different concentrations, indicating a significant reduction in pathogen growth.13 Azardiachta indica was also shown to be effective against A. solani.14 Mycelial growth of different species of Fusarium was inhibited by different concentrations of Convolvulus arsinoides and C. plurlictis plant extracts15 Allium cepa16 Adhatodavasica Azadirachta indica Cinnamomum camphora and Ocimum sanctum17 Ocimumbasilicum, Eucalyptus amygdalina, Ailanthus Excels und Lantana camera.18 In this study, extracts of all plant species belonging to different families were found to have significant levels of antifungal activity against Fusarium oxysporum.
Conclusion
Results of the present study indicated that all selected plant extracts showed antifungal activity against fusarium oxysporum f.sp. cumini. Extracts prepared with acetone and cow urine showed excellent antifungal activity and were able to inhibit the growth of Fusarium oxysporum f.sp. Cumini, which is responsible for cumin wilting. These preliminary results, obtained from in vitro experiments, may be supplemented by other more comprehensive studies in the open field (in vivo). From this research, it has been concluded that certain plant extracts are a good source of cost-effective and non-hazardous approach against Fusarium oxysporum f.sp. cumini. Plant extracts such as Psidium guajavam, Triumfetta Pilosa, Senna alexandrina, and Azadirachta indica have good antifungal efficacy, which may be used for formulating new, safer, and eco-friendly natural fungicides. This work has allowed selecting the best plant which may be used as a Phyto fungicide to control crop diseases, with the ultimate goal of developing a green alternative to synthetic fungicides.
Acknowledgment
The authors are thankful to the Atmiya University of Rajkot, for providing the facility to carry out this work. We also thank Dr. L. F. Akbari, Plant Pathologist, Agriculture University, Junagadh, for guidance and assistance.
References
- Strange, N., & Scott, P. R. (2015). Plant disease: a threat to global food security. Annual review of phytopathology, 43, 83–116.
CrossRef - Banerjee, , & Sarkar, P. K. (2003). Microbiological quality of some retail spices in India. Food Research International, 36(5), 469-474.
CrossRef - Devi Priya, B., &Thyagarajan, M. (2020). An investigation on production and productivity export performance of significant spices in the Country of India. Indian Journal of Science and Technology, 13(48), 4699-4707.
CrossRef - Contreras, D., Chamorro, A., & Wilkinson, S. (2020). The spatial dimension in the assessment of urban socio-economic vulnerability related to geohazards. Natural Hazards and Earth System Sciences, 20(6), 1663-1687.
CrossRef - Talaviya, J. R., & Jadeja, K. B. (2015). Efficacy of bioagents alone and in combination with microbial population against the wilt incidence of cumin. Journal of Biological Control, 29(3), 162-166.
CrossRef - Patel, N., Prasad, N., Mathur, R. L., & Mathur, B. L. (1997). Fusarium wilt of cumin. Current Science, 26(6), 181-182.
- Gamble, J. S. (1921). Flora of the Presidency of Madras. West, Newman, and Adlard.
- Singh, R. K., & Dwivedi, R. S. (1987). Effect of oils on Sclerotium rolfsii causing foot-rot of Indian Phytopathology, 40(2), 531-33.
- Shrivastava, A., Rizvi, G., Paijwar, M. S. (2011) Antifimgal activity of some wild medicinal plants against the growth of Fusarium oxysporum f.sp. cumini.
- Shivapratap, H. R., Philip, T., & Sharma, D. D. (1996). In vitro antagonism of Trichoderma species against mulberry leaf spot pathogen, Indian Journal of Sericulture, 35(2), 107-110.
- Singh, R. K., & Dwivedi, R. S. (1997). Studies on biological control of Sclerotium rolfsiiSacc causing foot rot of barley. Acta Botanica Indica.
- Alam, S., Alam, M. S., & Mahal, F. (1999). Growth inhibition (in vitro) of chili fruit rot pathogen Alternaria tenuis. Asiat. Soc. Bangladesh, Sci, 25, 211-216.
- Kumar, A., Rahal, A., Chakraborty, S., Tiwari, R., Latheef, S. K., &Dhama, K. (2013). Ocimum sanctum (Tulsi): a miracle herb and boon to medical science-A Int J Agron Plant Prod, 4(7), 1580-9.
- Maya, C., &Thippanna, M. (2013). In vitro evaluation of ethnobotanical important plant extracts against early blight disease (Alternaria solani) of tomato. Global Journal of Bioscience and Biotechnology, 2, 248-252.
- Furgal Wegrazyeka, (1984). CzecazeszytyNaukoweAkademi, Rolincz, Technizejw. Olsztytie. Ralnictwo, 39, 137-153.
CrossRef - El-Shami, M. A., Fadl, F. A., Sirry, A. R., & El-Zayat, M. M. (1985). Antifungal properties of garlic and clove juice compared with fungicidal treatment against Fusarium with Egyptian Journal of Phytopathology, 17, 55-62.
- Krishna, A., Prasad, A., & Ojha, N. L. (1986). Antifungal evaluation of leaf extracts for the control of some Cucurbitaceae fruit rot diseases. Indian Phytopathol, 39, 152-162.
- Bansal, K., & Gupta, R. K. (2012). Evaluation of plant extracts against Fusarium oxysporum, wilt pathogen of fenugreek. Indian Phytopathology.

