Introduction
Faba bean (Vicia faba L.) and chickpea (Cicer arietinum L.) are cool-season seed legume crops cultivated in the highlands of Ethiopia. Faba bean and chickpea cover 27 and 13% of the total area and 31 and 12% of the total production of pulses, respectively (CSA)1. They also have significant contribution in securing human and animal nutrition in the country. Their respective yields, 21 kg ha-1 and 16 kg ha-1, are significantly lower than their attainable yields (CSA)1. However, their net profit per ha has been found to be higher than most cereals (Yirga)2 which is highly attributed to the extensive management. This is attributed to the saving of 150-200 kg ha-1 of N as well as some 20-50 kg in the subsequent crop. However, the current national productivity of faba bean and chickpea (2.1 and 1.6 t ha-1 (CSA)1 are far below the verified attainable yields of 4.1 t ha-1 and 5.3 t ha-1, respectively (Fikrie)3 due to inadequate nutrient use (Tsige and Hailemariam)4.
Root and foliar diseases like mildew and rust (faba bean) and the wilt/root-rot complex(chickpea) reduce attainable, if not potential, yield in most part of Ethiopia to a considerable degree (Schneider and Anderson)5. Yield losses were estimated to be 50% under severe epidemic conditions (Abrham)6. Most farmers, seed companies, farmer cooperatives or unions overcome the seed-borne diseases with fungicide seed treatment (personal observations). On the other hand, rhizobial inoculation to grain legumes has been adapted as an affordable technology to sustainably enhance faba bean and chickpea productivity in Ethiopia. Improvement of average seed yield of grain legumes by 10% on 60-80% of the inoculated farms (Mitiku and Mnalku7, Mnalku and Mitiku8) was documented. Unfortunately, the available rhizobial inoculants (RI) in Ethiopia has powder formulation and used in dressing on to the seed just before planting (Mnalku)9. In view of this, users often raise their concerns whenever they plan to dress fungicides and RIs on same seed and time.
Plenty of researches have been carried out on the compatibility of RIs and fungicides during joint use (co-dressing). However, the majority was on in-vitro conditions and yet had controversial outputs due to variation in methods and the lack of quantitative data (Curley and Burton)10. The recommendations obtained so far were either conflicting or conditionals. Some reports revealed the antagonistic effects of fungicides on the viability of rhizobia, on nodulation and seed yield (Zilli)11 while applied at higher rates of applications (Ahmed and Khan)12. In contrast, non-antagonistic or synergistic effects were also reported from other studies (Periera)13 but hypothesized to be relied on the fungicide (type, rate and time of dressing with respect to the rhizobia) (Jinturkar)14 and strain (Kutcher)15.
Despite fungicide and RI-based seed treatments have been components of a seed legume production package in Ethiopia, the importance of their apparent interaction effect on the nodulation and productivity of grain legumes has not been subjected to research. As a result, contextual evidences that help advise the farmers were extremely lacking. Therefore, the objectives of this study were to investigate the effect of (i) fungicides on mesorhizobia and chickpea nodulation under Vertisol condition and (ii) fungicides on rhizobia, faba bean nodulation and seed yield under Nitisol condition.
Materials and Methods
Description of Experimental Situation
The studies were conducted on the station of Holeta Agricultural Research Center (HARC), Ethiopia during the 2016 and 2017 main rain seasons. The station is found at 40 km west of Addis Ababa and located at longitude, latitude and altitude of 9.0591 N, 38.5045 E and 2405 meters above sea level (masl), respectively. The faba bean experiment was carried out at field while that of chickpea was conducted on potted soil under green house at Holeta. The respective soil types were Nitisol and Vertisol and their typical chemical properties are showed on Table 2. Both soils types were found in the central highland of Ethiopia and have been under faba bean-potato-cereals and chickpea-teff/wheat-chickpea crop rotation schemes, respectively. The rainfall received during the effective growing months in both seasons was shown in Figure 1. Composite soil samples were collected and analysed at Holeta soil laboratory using the standard methodologies.
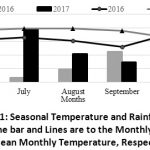 |
Figure 1: Seasonal Temperature and Rainfall Records. |
Description of the Materials
The RI of EAL-029/CP-029 (chickpea), and FB-1017 and FB-1035 (faba bean) are indigenous elite strains (AKLDP)16. All inoculants were prepared following the standard protocol at Holeta Agricultural Research Center (Mnalku)9. Likewise, the three pesticides used in the study are the most available and commonly used fungicides and had major characteristics mentioned as follows:
Table 1: The Major Characteristics of the Three Fungicides Used in the Study.
| Fungicide | Type | Rate | Status |
| Apron Star 42 WS | Fungicide-insecticide | 2.5 g/kg seed | Registered |
| Imidalm T450 WS | Fungicide-insecticide | 0.75 g/kg seed | Registered |
| Mancozeb 80WP | Fungicide | 2.5 kg/ha | Registered |
Gabelcho (faba bean) and Arerti (chickpea) are released varieties (MoANR)17 and their seeds were obtained from Holeta agricultural research center.
Experimental Design and Treatments
In case of chickpea, the below indicated treatments were laid in RCB design with three replications. Seeds of Arerti were dressed with EAL-029, Apron Star, and Imidalm according to the treatments (Table 2) and planted in the potted soil but thinned to 5 after 7 days. Strain EAL-029 was dressed at 3.12g kg-1 of Arerti seed (Mitiku)18. Nodulation and biomass data were collected at the 45th day after emerging.
Table 2: List of Treatments with Respective Acronyms for Both Crops.
| # | Chickpea | Faba bean (dressing) | Faba bean (Spray) |
| 1 | Apron Star then EAL-029 ( AtE) | Apron Star + FB-1017 (A17) | Mancozeb + FB-1017 (M17) |
| 2 | Apron Star + EAL-029 (AE) | Apron Star + FB-1035(A35) | Mancozeb + FB-1035 (M35) |
| 3 | Imidalm then EAL-029 (ItE) | Apron Star + 18 kg N ha-1 (AN) | Mancozeb +18 kg N ha-1(MN) |
| 4 | Imidalm + EAL-029 (IE) | FB-1017 | FB-1017 |
| 5 | Apron Star + Imidalm then EAL-029(AItE) | FB-1035 | FB-1035 |
| 6 | Apron Star + Imidalm + EAL-029 (AIE) | ||
| 7 | EAL-029 (control) (E) |
“+” = simultaneous dressing otherwise the pesticide precedes in a week time.
In case of faba bean, composite soil samples were taken at 0-20 cm depth before planting for determination of major properties. The plot length and width were 3 and 4 meters, respectively. Spacing between plots and blocks were 0.5 and 1 meter, respectively. Inter and intra row spacing were maintained at 0.4 and 0.1 meters, respectively. Apron Star and/or inoculant dressed (same to chickpea above) Gabelcho variety seed was sown at 200 kg ha-1. The treatments were replicated four times and laid out in RCB design. On the 30th day after sowing, Mancozeb was sprayed at a rate of 2.5 kg ha-1 regardless of the natural occurrence of the target disease. Care was taken to protect non-target plots from spray drift. Control plots were sprayed with tap water. Thinning, weeding, P fertilization of faba bean were done in uniform condition to all plots as per the recommendation of (Agegnehu and Demissie)19.
Data Management
Average active nodule number per plant (NC) and seed yield data were recorded at peak flowering and physiological maturity (harvesting), respectively on plot basis. Square root transformed NC following O’Hara and Kotze 20, seed yield (SY) (adjusted to 9% seed moisture content), nodule weight (NW) and shoot biomass yield (SBY) were tested for normal distribution and homogeneity of variance of residuals by diagnostic plots generated by PROC UNIVARIATE and PROC GPLOT in SAS-STAT software. Analyses of variance (ANOVA) for the effects of treatments on nodulation, and yield were made separately for each year, because of considerable variation in the nature of response in different years. All analyses were performed using statistical package SAS system, version 9.2. Least significant difference (LSD) values were used to separate differences among treatments means at 5% probability level.
Results and Discussions
Experimental Soil Test
Major soil chemical properties were determined (Table 2) and thus the soil pH of main station of Holeta Agricultural Research Center (HARC) and Vertisol of Ginchi were rated as very strongly acidic and moderately acidic (Hazelton and Murphy)21, respectively. The Vertisol is appropriate for the production of most field crops whereas liming would be essential on the Nitosol to improve inoculant performances (Mitiku and Mnalku)7, and pH and faba bean seed yield (Agegnehu)22. Note that faba bean is moderately sensitive to low pH, growth is reduced at pH < 6. Soil organic carbon and total N were rated moderate for both locations (Hazelton and Murphy)21. Therefore, a response to inoculation is expected as soil N level would not inhibit nodule formation or N fixation (Kagan and Kayan)23. The soil C:N ratios of both locations were between 7.7-10.1, which is favorable range for decomposition of organic materials and subsequent release of N and other essential nutrients in the tropical farming systems (Swangjang)24.
Table 3: Major Physicochemical Properties of Study Soils During 2016 Growing Season.
| Parameters | Potted Vertisol | HARC Nitisol | Test methods |
| pH | 6.02 | 4.69 | 1:2.5 H2O |
| Available P (ppm) | 14.19 | 4.72 | Bray II |
| OC (%) | 1.23 | 1.29 | |
| Total N(%) | 0.16 | 0.14 | |
| C:N | 7.7 | 9.21 | – |
Like the pH, the average concentration of available P differed between the experimental soils. The Nitosol had very low (0-8 mg P kg−1 of soil) while the Vertisol had low to optimum (9-20 mg P kg−1 of soil) available P ratings (Mallarino)25. As P source is desirable to appreciate rhizobial survival and establish effective symbiosis with the host legume (Fatima et al.)26, the very low to medium available P ratings would require P application based on the climate, and P use efficiency of the target legume species (Graham and Vance)27. That is why 20 kg P ha-1 was added to both soils of the current study.
Co-Dressing of Chickpea Seed with Rhizobia and Fungicides
The study revealed non-significant difference (P ≤ 0.05) between the treatments in nodule dry weight, nodule number and shoot dry weight (Table 3). The implication is that simultaneous dressing of chickpea seed with Apron Star and/or Imidalm fungicides at respective rates of 2.5 and 0.75 g kg-1 seed, along with chickpea local rhizobial inoculant (EAL-029) caused little or non-significant harm on NW, NC and shoot dry weight (SW) of chickpea as compared to the EAL-029 (check) (Table 3). Similar findings were reported by Kyei-Boahen28, where Apron Star did not cause significant effect of on NC, NW and SDW of chickpea and hence its compatibility with the chickpea rhizobial inoculum under controlled environment. Muthoni29 confirmed the beneficial effect of cupper oxychloride and rhizobia combination on common bean, green gram and lablab in terms of root rot disease management, and NC and SDW enhancement at green house. Kaur30 also reported the compatibility of Captan with soybean rhizobia in NC and nitrogen fixation at field level in the labeled rate.
Table 4: Influence of Joint Seed Dressing of Apron Star and/or Imidalm Fungicides, with EAL-029 on Chickpea under Potted Vertisol.
| Treatments | NC (# plant-1) | NW (g plant -1) | SW (g plant-1) |
| Apron Star then EAL-029 ( AtE) | 88 | 0.13 | 3.35 |
| Apron Star + EAL-029 (AE) | 55 | 0.07 | 3.09 |
| Imidalm then EAL-029 (ItE) | 59 | 0.07 | 3.19 |
| Imidalm + EAL-029 (IE) | 58 | 0.09 | 3.08 |
| Apron Star + Imidalm then EAL-029 (AItE) | 77 | 0.12 | 2.97 |
| Apron Star + Imidalm + EAL-029 (AIE) | 56 | 0.07 | 2.92 |
| EAL-029 (control) (E) | 60 | 0.08 | 3.55 |
| CV (%) | 39 | 30 | 20.38 |
| LSD(P ≤ 0.05) | ns | ns | ns |
Though non-significant, AtE and AItE displayed positive NC and NW increment over the sole inoculant (Figure 2). Accordingly, AtE changed NC, NW and SW of chickpea by 47 (+), 63(+) and 6(-)% over the sole inoculant, respectively. This tends to reveal the importance of sequentially dressing (7 days in our case) of Apron Star and then the inoculant. The significance of the sequential dressing particularly on the inoculant survival has been well noted by Van Kessel and Hartley 31 and Gaur et al.32. The allowance of such span of time in between the fungicide seed treatment and the inoculant would make the fungicide to dry and hence prevent any antagonistic reaction as hypothesized by Henson et al (unpublished). Cheema33 also confirmed the absence of adverse effects on germination, nodulation, yield-attributing characters and SY of chickpea when Captan (3g kg-1) and endosulfan (35 EC@10 ml kg-1) treated seeds were kept for overnight and dressed with Rhizobium (Strain LRG 33) just before sowing.
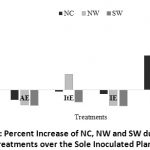 |
Figure 2: Percent Increase of NC, NW and SW due to the Treatments over the Sole Inoculated Plant. |
Inter-fungicide compatibility difference was also observed in the current study. In view of this, Apron Star appeared to be more compatible with EAL-029 and the symbiotic process than Imidalm (Figure 2). Similarly, Captan and Carbendazim showed compatibility difference to soybean (Kaur)30. Beyond the constituent difference, the slightly higher rate of Imidalm would have its own contribution.
Co-Dressing of Faba Bean Seed with Apron Star and Rhizobia
The result of effect of FB-1017 and FB-1035 along with Apron Star seed dressing on NC and yield parameters are tabulated in table 4. The result indicated that none of the treatments revealed significant inferior (P≤0.05) mean of responses to the sole inoculants. This denotes that Apron Star was highly compatible with faba bean rhizobial strains as none of the traits were adversely affected by the treatment. Instead, A17 (Apron Star + FB-1017) remarkably improved the SBY and SY of faba bean as compared to the sole FB-1017 or the AN during 2016 growing season. The current finding is in agreement with the report of Bulyaba51 which underscored the non-significant effects of fungicide (ApronMaxx) seed treatment at 3.3 mL kg-1 seed about 2 weeks before planting and Bradyrhizobium inoculation on soybean and cowpea. Field pea has also been shown to benefit from the use of Rhizobium inoculation and seed-applied fungicides under intensive production (Kutcher)15.
The importance of environment (climate and/or soil) with regards to fungicide-rhizobia interaction was noted. The augmentation of the synergistic interaction effect of Apron Star and FB-1017 during 2016 marked the importance of growing season (climate and/or soil) on the determination of fungicide-rhizobial inoculant interaction status and strength. Regardless of the temperature, the optimum amount and appropriate distribution of rainfall during July to October (the actual growing periods of faba bean in the study area) (Figure 1) was supposed to attribute to the improved synergy. In this regard, summer season was noted to enhance such compatibility as compared to Rhizobium alone (Jintukar)14.
Table 5: Effect of Rhizobia and Apron Star seed Co-Dressing on Symbiotic Traits of Faba Bean in 2016 and 2017.
| Treatment | NC(# plant-1) | NW (g plant-1) | SBY (kg ha-1) | SY (kg ha-1) | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| A17 | 4.3 | 13 | 1.07 | 0.5 | 7389a | 4074 | 3033a | 1363 |
| A35 | 2.7 | 25 | 1.03 | 0.9 | 7361a | 4555 | 2962a | 1526 |
| AN | 3.6 | 25 | 1.04 | 1.0 | 6111b | 4259 | 2650ab | 1330 |
| FB-1017 | 3.3 | 25 | 1.02 | 0.9 | 6222b | 4444 | 2477b | 1552 |
| FB-1035 | 1.6 | 22 | 1.02 | 0.70 | 6917ab | 4037 | 2734ab | 1278 |
| CV(%) | 34.4 | 33 | 4.13 | 22 | 8.38 | 9 | 8.2 | 9 |
| LSD (P<0.05) | ns | ns | ns | ns | 1033 | ns | 411 | ns |
| Year | ||||||||
| 2016 | 1.78b | 1.05a | 6778a | 2752a | ||||
| 2017 | 4.54a | 0.87b | 4241b | 1386b | ||||
| CV(%) | 36 | 15.8 | 10.5 | 12.45 | ||||
| LSD (P<0.05) | 0.78 | 0.110 | 399 | 178 | ||||
SBY= Shoot biomass yield
We clearly observed that year (growing season + soil) caused significantly (P≤ 0.05) different NC, NW, SBY and SY of faba bean. Accordingly, 2016 growing season had superior NW, SBY and SY to 2017 growing season whereas the reverse was true in NC case. This confirmed that high nodulation will not essentially associate with higher nodule mass or yield. The interspecific competition among the numerous nodules (bacteriods) for resources would have affected the weight as well as the subsequent symbiotic performances.
 |
Figure 3: Percentage Increase in Symbiotic Traits of Faba Bean in Response to Co-Application of Apron Star with FB-1017 and FB-1035 in 2016 and 2017 Growing Seasons. (Relative to Sole Inoculants) |
According to Figure 3, the relative performances of Apron Star-rhizobia seed dressing were variable depending on the strain and the growing season. All relative performances of Apron Star-rhizobia strain were positive in 2016 (absolute superiority of the co-inoculation over the sole inoculation). In 2017, however, the performances of A17 showed negative relative performances despite statistically unjustified. Interestingly, A17 and A35 showed 22 and 19 % seed yield increment over their respective sole inoculants in 2016 and 2017 growing seasons, respectively. This verifies the season specific compatibility of both combinations. But, when averaged by year, they displayed 10 and 14% seed yield improvement over their sole inoculant. This would confirm the stable compatible performance of Apron Star-FB-1035 across growing seasons. Similar determinant role of strain genotype with regards to compatibility of fungicide-strain interaction was noted by Guene.33.
The overall interaction of Apron Star-faba bean rhizobia seed dressings were mostly synergistic. Such positive interplay between seed-applied fungicide and Rhizobium inoculant suggests the indispensability of the joint use of fungicide seed treatment with Rhizobium inoculant for productivity improvement of faba bean even under low disease risk conditions. Aynalem and Assefa35 hypothesized that buffering and nutritional effects of some fungicides would benefits the rhizobial isolates. On the other hand, the few negative interactions between seed-applied fungicides and Rhizobium inoculants suggests that the use of fungicide seed treatment with Rhizobium inoculant may be considered when disease development is likely due to conducive environmental conditions (moist, humid and warm) or high inoculum levels (seed or soil history) (Kutcher)15.
Effect of Mancozeb Spray on Symbiotic Traits of Inoculated Faba Bean
As table 5 depicts, the fungicide spray-rhizobial inoculation treatments did not show significantly (P≤0.05) different NC, NW, SBY (in both years), and SY (in 2016) of faba bean. However, SY in 2017 showed significant differences, where FB-1035 gave lower seed yield than MN and FB-1017. When averaged by treatments (year effect), all the measured parameters displayed very highly significant differences between the years. Even if data was not shown, crossover interaction effects of fungicide spray-Rhizobium inoculant treatments and years could reveal real differences, where M17 and M35 gave 6 and 8 % more garin yield of faba bean during 2016 and 2017 growing seasons, respectively. When averaged, however, both combinations had 4% increment over their sole inoculants. The effect of post emergence (30 days after sowing) foliar spraying of mancozeb at the labeled rate to inoculated faba bean plant was not harmful with regards to rhizobia establishment, nodulation and yield.
Table 6: Effect of Rhizobia and Mancozeb Spray on Symbiotic Traits of Faba Bean in 2016 and 2017 Seasons.
| Treatment | NC (# plant-1) | NW(g plant-1) | Shoot biomass yield (kg ha-1) | Seed yield (kg ha-1) | ||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| M17 | 1.96 | 23 | 1.04 | 0.71 | 6111 | 4592 | 2616 | 1526ab |
| M35 | 1.25 | 31 | 1.05 | 0.75 | 6861 | 4370 | 2730 | 1377ab |
| MN | 1.02 | 23 | 1.04 | 0.73 | 5889 | 4593 | 2539 | 1567a |
| FB-1017 | 2.25 | 23 | 1.02 | 0.92 | 6222 | 4444 | 2477 | 1552a |
| FB-1035 | 2.34 | 24 | 1.02 | 0.7 | 6917 | 4037 | 2734 | 1278b |
| CV(%) | 41 | 25 | 3.5 | 17.4 | 7.5 | 7 | 10.1 | 10 |
| LSD (P<0.05) | ns | ns | ns | ns | ns | ns | ns | 271 |
| Year | ||||||||
| 2016 | 1.4b | 1.05a | 6444a | 2625a | ||||
| 2017 | 4.6a | 0.85b | 4352b | 1428b | ||||
| CV(%) | 34 | 13.59 | 11.59 | 15.1 | ||||
| LSD (P<0.05) | 0.7 | 0.08 | 432 | 211 | ||||
The fact that mancozeb is a contact fungicide and often sprayed later with relative to the usual nodule initiation and development period of faba bean (O’Hara)36 would bring spatial and temporal divergence between mancozeb and rhizobia. Such instances will make it unlikely to get adversely affected rhizobia, nodule number and nodule dry weight. That is why the NC and NW responses were lacking treatment-driven real differences regardless of strain genotype and growing seasons. The statistical similarities between the treatments and the respective controls (sole inoculants) in SBY and SY responses, still confirmed the absence of adverse effects of mancozeb in nitrogen fixation, biomass accumulation and seed filling processes in the later growth stages. Though not consistent across seasons and strains, mancozeb spray showed synergy with rhizobia in few cases of SBY and SY. Stimulation of rhizobia in reaction to fungicides spray stress was reported in studies. In general, fungicides improved the health of seedling by providing better absorption of nutrients and thereby providing more area to rhizobia to infect the roots and formation of nodules.
Conclusion
The study revealed that joint dressing of Apron Star or/and Imidalm with rhizobial strain EAL-029 on chickpea (Arerti) seeds under Vertisol of Ginchi caused insignificant reduction in nodulation, biomass accumulation and seed yield. Dressing of Apron Star a week right before rhizobial preinoculation showed synergetic effect on grain yield of chickpea. The nodulation and yield of faba bean (Gabelcho) variety were not substantially reduced up on co-dressing of Apron Star and rhizobial inoculants to the seed or spraying of mancozeb along with FB-1035 or FB-1017 preinoculation. The good synergy, however, varies with the growing seasons. The chickpea outputs yet require verification at field level. But farmers may consider the joint use Apron Star or Mancozeb along with rhizobial isolate to improve the productivity of faba bean at least in similar agroecologies.
Conflict of Interest
Authors declare no conflict of interest
Acknowledgements
The authors are thankful to Ethiopian Institute of Agricultural Research for the financing the research project and to Mr Birhan Abdulkadir for his unreserved push and guidance in writing.
References
- CSA (Central Statistical Agency). 2018. Agricultural sample survey 2017/2018 (2010 E.C.), Volume I Report on Area and Production of Major Crops (Private Peasant Holdings, Meher Season). Statistical Bulletin 586, April 2017, 14-15.
- Yirga, C., S. Rashid, B. Behute and S. Lemma. 2010. Pulses value chain potential in Ethiopia; Constraints and opportunities for enhancing exports”, International Food Policy Research Institute (IFPRI), Addis Ababa, Ethiopia.
- Fikre, A. Unraveling valuable traits in Ethiopian grain legumes research hastens crop intensification and economic gains: A review. Universal Journal of Agricultural Research 2016; 4: 175-182.
CrossRef - Tsige, A. and A. Hailemariam, 2006. Biological nitrogen fixation research on food legumes in Ethiopia, Food and forage legumes of Ethiopia progress and prospects, ICARDA:172-176.
- Schneider, K. and Anderson, L. 2010. Yield gap and productivity potential in Ethiopian agriculture: Staple Grains & Pulses, EPAR Brief No. 98. University of Washington:1-24.
- Abrham, T., 2008. Increasing crop production through improved plant protection – Volume I. Plant Protection Society of Ethiopia (PPSE), 19-22 December 2006. Addis Ababa, Ethiopia. PPSE and EIAR. 589-590.
- Mitiku, G. and Mnalku, A. Faba Bean (Vicia faba ) yield and yield components as influenced by inoculation with indigenous rhizobial isolates under acidic soil condition of the central highlands of Ethiopia. Ethiop. J. Agric. Sci. 2019; 29: 49-61.
- Mnalku, A. and Mitiku, G. Response of chickpea (Cicer arietinum) to indigenous rhizobial isolates inoculation on Vertisol of central Ethiopian highland. Ethiop. J. Agric. Sci. 2019; 29(2):109-117.
- Mnalku, A., Demissie, N. Muleta, D. Abera, Y. and Mitiku, G. 2019. Manual for rhizobial inoculant development and management. Ethiopian Institute of Agricultural Research. 51-52.
- Curley, R.L. and Burton, J.C. Compatibility of japonicum with chemical seed protectants. Agronomy Journal 2020; 67 (6).
CrossRef - Zilli, J.E., Ribeiro, K.G., Campo, R.J. and Hungria, M. Influence of fungicide seed treatment on soybean nodulation and seed yield. Bras. Ci. Solo. 2009; 33: 917-923.
CrossRef - Ahmad, M. and Khan, M.S. Pesticides as antagonists of rhizobia and the legume-Rhizobium symbiosis: A paradigmatic and mechanistic outlook. Biochemistry & Molecular Biology 2013; 1(4):63-75.
CrossRef - Pereira, C.E., de Souuza, F.M., Oliveira, J.A. and Caldiera, C.M. Compatibility among fungicide treatments on soybean seeds through film coating and inoculation with Bradyrhizobium Acta Scientiarum Agronomy 2010; 32 (4):585-589.
CrossRef - Jinturkar, B.P. Compatibility between groundnut Rhizobium and seed dressing fungicide. International Journal of Curr. App. Sci.2017; 6(5):1067-1075.
CrossRef - Kutcher, H.R, Lafond, G., Johnston, A.M., Miller, P.R., Gill, K.S. and May, W.E. Rhizobium inoculant and seed-applied fungicide effects on field pea production. J. Plant. Sci.2002; 82: 645–661.
CrossRef - AKLDP (Agriculture Knowledge, Learning Documentation and Policy) Project. 2016. Improving crop yields in Ethiopia: Early impacts from rhizobia-inoculated legume seed. Technical Brief. 5-6.
- MoANR (Minstry of Agriculture and Natural Resources). 2016. Crop variety register Issue No. 19, Addis Ababa, Ethiopia :107-108.
- Mitiku, G., Mnalku, A. and James, W. 2018. Application guideline for rhizobial biofertilizer technologies. Ethiopian Institute of Agricultural Research (EIAR), Addis Ababa, Ethiopia:12-13.
- Agegnehu, G. and Demissie, M. Effect of seed size, planting density and phosphate fertilizer on yield and yield components of faba bean in the central highlands of Ethiopia. J. Agric.Sci. 2011; 21: 95-107.
- O’Hara, R.B. and Kotze, D.J. 2010. Methods in ecology and evolution: Do not log-transform count data, 1:118-122.
CrossRef - Hazelton, P. and Murphy, B. 2016. Interpreting soil test results: What do all the numbers mean?” CSIRO publishing: 83-100.
CrossRef - Agegnehu, G., Yirga, C. and Erkossa, T. 2019. Soil acidity management. Ethiopian Institute of Agricultural Research. Addis Ababa, Ethiopia, pp: 24-31.
- Kagan, S.and Kayan, N. The influence of inoculation and nitrogen treatments on yield and yield components in chickpea ( arietinum L.) cultivars. Legume Res. 2014;37:363-371.
CrossRef - Swangjang, K., 2015. Soil carbon and nitrogen ratio in different land uses. International Conference on Advances in Environment Research.
- Mallarino, A.P., Sawyer, J.E. and Barnhart, S.K. 2013. A general guide for crop nutrient and limestone recommendations in Iowa. Department of Agronomy, Iowa State University, Iowa.
- Fatima, Z., Zia, M. and Chaudhary, F. Effect of Rhizobium strains and phosphorus on growth of soybean (Glycine max) and survival of Rhizobium and P solubilizing bacteria. J. Bot.2006; 38:459-464.
- Graham, P. and Vance, C. Nitrogen fixation in perspective: an overview of research and extension needs. Field Crops Res.2000; 65:93-106.
CrossRef - Kyei-Boahen, S., Slinkard, A.E and Walley, F.L. Rhizobial survival and nodulation of chickpea as influenced by fungicide seed treatment. J. Microbiol. 2001;47:585-589.
CrossRef - Muthomi, J.W., Otieno, P.E. Chemining’wa, G.N. Nderitu, J.H. and Wagacha, J.M. Effect of legume root rot pathogens and fungicide seed treatment, nodulation and biomass accumulation. Journal of Biological science 2007; 7(7): 1163-1170.
CrossRef - Kaur, C., Maini, P. and Shukla, N.P. Effect of Captan and Carbendazim fungicides on nodulation and biological nitrogen fixation in soybean. Asian J. Exp. Sci. 2007; 21(2): 385-388.
- Van Kessel, C. and Hartley, C. Agricultural management of seed legumes: has it led to an increase in nitrogen fixation? Field Crops Res.2000; 65:165-181.
CrossRef - Gaur, P.M., Tripathi, S. C., Gowda, L.L., Ranga, R.G.V., Sharma, H.C., Pande, S., Sharma, M. 2010. Chickpea seed production manual. Patancheru 502 324, Andhra. Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics: 28-29.
- Cheema, H.K., Sharma, P., Singh, R., Taggar, K.G., Khanna, V. and Kooner, B.S. Efficacy and compatibility of insecticides, fungicide and Rhizobium inoculant in combination for seed treatment in chickpea ( arietinum). Indian Journal of Agricultural Science 2009; 79(3):190-194.
- Guene, N., Diaw, F., Diaw, D., Adama, D. and Gueye, M. Nodulation and nitrogen fixation of field grown common bean (Phaseolus vulgaris) as influenced by fungicide seed treatment. African Journal of Biotechnology 2003; 2 (7):198-201.
CrossRef - Aynalem, B. and Assefa, F. Effect of glyphosate and mancozeb on the rhizobia isolated from nodules of Vicia faba and on their N2-fixation, North Showa, Amhara Regional State, Ethiopia. Advances in Biology 2017; 1-7.
CrossRef - O’Hara, G.W., Hungria, M., Woomer, P. and Howieson, J.G. 2016. Field experiments with rhizobia. In Working with rhizobia, Eds., Howieson, J.G. and M.J. Dilworth. Canberra: Australian Centre for International Agricultural Research :265-269.


