Introduction
Herbal medicines have gained worldwide attention for their use in treating chronic diseases due to their higher effectiveness and less side effects than those of synthetic drugs. Rubus is a genus that belongs to the Rosaceae family, which grows naturally in the mountainous forests of Indonesia. It has a high potential to be developed as crop cultivated plant. In fact, Rubus is one of the largest genera within Rosaceae that consists of 740 species. This plant is also native to six continents and can grow in several locations. It is reported that 46 species of Rubus were found in Malesia Regionand 25 speciesin Indonesia.1,2 R. fraxinifolius, belonging to the Sub genus Malachobatus, is one of Raspberry, which is distributed in mountainous forests of Indonesia. Kalkman (1993)1 reported that this species was found in Borneo, Java, Celebes, Moluccas, Lesser Sunda Islands, atanaltitude of 0-2500 meter abovesea level. Furthermore, Cibodas Botanical Garden, an institute of ex situ conservation, has collected eleven Rubus species some of which are from mountains in Indonesia3 included R. fraxinifolius,.4
The traditional medicinal use of Rubus in South east Asia was very similar to the one in Europe. Some Rubus were reported to produce various substances that possess pharmacological effects, including antidiabetic, antibacterial, anti-inflammatory, antioxidant, antidiarrheal, anti-tumor, anti-fertilization, neuroprotective and wound healing properties.4,5
The drying process is an efficient way to maintain the phytochemical content in plant especially for medicinal herbs.6 Furthermore, drying can help reduce the cost of the final product since product weight determines transportation and storage costs6. The biological activity of Rubus leaves has been previously reported 4, 6, furthermore, Abu Bakar (2016)7 reported the phytochemical content and activities of R. fraxinofolius fruits, while Shamsudin (2019)8 reported Antioxidant activities and phytochemicals content in three different parts (ie, fruit, stem and leaves) of R. fraxinofolius which dried with freeze dryers. However, still no study has ever been conducted on understanding the effect of drying R. fraxinifolius leaves on their phytochemical content and bio-activity. Therefore, the aim of this study is to evaluate the chemical compositions, expecialy is phenolic and flavonoid compounds of the selected young R. fraxinifolius leaves that underwent different drying processes by using qualitative analysis and biological properties such as antioxidant, antidiabetic, and antibacterial activities.
Materials and Methods
Plant Materials
The leaves of R. fraxinifolius were collected from Cibodas Botanical Garden (latitude: -6° 44’ 19.19” and longitude: 107° 00’ 12.00” E) on November 2017. R. fraxinifolius has compound leaves, with peduncle, rachis, pedicel, and leaflets. Leaf shoots were taken from the 1st to 3rd leaves in each particular branch. Leaflets had not properly opened and they were green and glowing on the upper surface but were reddish on the lower surface. The texture was very soft, so they could be easily cut by hand. Young leaves were taken from below shoot leaves av. 4th – 9th leaf (depending on the size of the branch) towards the base of the branch, leaflets were completely open, green and glowing, with a flush of red in some parts (lower surface and midrib of the leaflets, upper part of the rachis and pedicels), and the branch where the leaves attached was very firm. Mature leaves are located close to the base of the branch. They were green but did not glow; only the spines and a very small portion of the rachis were reddish, the texture was hard and the spines were sharp. Leaves were prepared in three different ways: fresh leaf, dried at room temperature (air drying), and dried at 50ºC (oven drying). The dried sample was placed in an airtight container until further use.
Chemical Reagents
Antioxidant reagent: DPPH (2,2-diphenyl-1-picrylhydrazyl), Follin-Ciocalteu Reagent, gallic acid, and quercetin were purchased from Sigma-Aldrich (St. Louis, USA). Vanillin, Na2CO3, AlCl3, NaNO2, NaOH, and H2SO4, were obtained from Merck (Darmstadt, Germany). α-Glucosidase assay: α-glucosidase [(EC 3.2.1.20)] type I from S. cerevisiae was purchased from Wako (Tokyo, Japan) and 4-nitrophenyl-Dglucopyranoside (PNPG) was purchased from Sigma (St. Louis, USA). Other commercially available chemicals were used as received and solvents were distilled before being used.
Extraction Method
The sample leaves of R. fraxinifolius (20 g) were macerated with methanol (250 mL x 3) at room temperature, and the solvent was evaporated in vacuo. The dried methanol extract was further used for biological activities assay and phytochemistry identification.
Qualitative Phytochemicals Screening Content of R. fraxinifolius Extracts
Qualitative phytochemical screening was carried by colorimetric methods to detect the presence of secondary metabolites in the extract using standard methods.9,10 Whereas for The total phenolic content was estimated according to Folin-Ciocalteu method11,12 and total flavonoid was determined according to aluminium trichloride method12,13 using quercetin as the reference compound.
Biological Activities Assay of R. fraxinifolius Extracts
The experiment methods for antioxidant was conducted by DPPH free radical scavenging effect and antidiabtes assay was determined based on α-glucosidase inhibitory activity were the same as described in our previous report.13
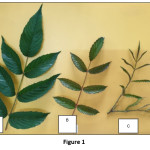 |
Figure 1 Click here to View Figure |
Determination of Antibacterial Activity
The antibacterial activities of the extracts were evaluated by disc diffusion
assay14 with slight modification. The antibacterial activity test was carried out by diffusion method of using 6 mm diameter paper discs with test bacteria (Staphylococcus aureus (InaCC-B4), Bacillus subtilis (InaCC-B334), and Escherichia coli (InaCC-B5)). The antibacterial activity test was carried out with three repetitions. 10 μL of each sample extract (5%) was pipetted into the filter disc on NA media that had been inoculated with test bacteria, then placed incubation was carried out at 37°C overnight. Streptomycin was used as a positive control, whereas DMSO was used as a negative control. Observations were made on the formation of inhibition zones around the paper disc.
Liquid chromatography-mass spectrometry-mass spectrometry (LCMS-MS)
Secondary metabolites from methanol extracts were determined using a LCMS-MS analysis, Xevo G2-XS QTof Waters MS Technologies (USA) equipped with an electrospray ionisation (ESI) source was coupled to an UPLC analysis was performed using a Waters Acquity Ultra Performance LC system. The analysis procedure was carried out in accordance with previously reported.15
Statistical Analysis
Data were analyzed using Microsoft Excel and reported as mean ± standard deviation of triplicate determination.
Results and Discussion
Drying is an important process in postharvest management and sales of herbs. Herbal drying is intended to inhibit microbial growth and retardation of enzymatic reactions that can prevent the stability of active compounds. However, it can also give rise to other changes that might affect the herb quality due to the loss of bioactive compounds, although some of the phytochemicals are more thermostable than others7. The effect of the fresh leaf (FL), air-dried (AD) and oven-dried (OD) samples of R. fraxinifolius Poir. young leaves were observed on qualitative, quantitative, and biological activities and they results were summarised in Table 1, 2 and 3. Although the phytochemical investigation revealed the presence of flavonoids, tannin, phytosterol, and phenolic compounds in all plant extracts (Table 1), quantitative analysis showed different amount of total phenol and flavonoid in all samples (Table 2). In our study, FL showed the highest phenolic and flavonoid contents of 25.35 ± 1.06 mg GAE/g and 28.71 ± 2.07 mg QE/g extract, respectively, whereas AD and OD had lower concentration of phenolic and flavonoid compounds (Table 2). It might be because drying caused reduction in phenolic and flavonoid contents in leaves. This finding was consistent with several research reports6 that oven drying could degrade some phytochemicals, such phenolic compounds from plants. This could mean that these compounds were sensitive to drying treatment.
Previous studies have indicated that the drying temperature led to a notable decline in phytochemicals.6 This could be due to the intense and prolonged drying that resulted in enzymatic degradation of the phytochemicals.16 Both total phenolic and flavonoid contents obtained in this study were relatively lower than those of leaf extract of R. fraxinifolius tested in previous studies,4 which was extracted using Soxhlet apparatus by n-hexane, ethyl acetate, and methanol. However, it was slightly higher than the total phenolic and flavonoid contents of R. moluccanus L., R. fraxinifolius Poir., and R. alpestris Blume.7
Table 1: Yield and qualitative phytochemical screening of extracts
| Extract | Yield of extract (%) | Metabolites | ||||||
| Flavonoid | Alkaloid | Steroid | Terpen | Tanin | Saponin | Quinon | ||
| FL | 10.96 | + | – | + | – | + | + | + |
| AD | 15.56 | + | – | + | – | + | + | + |
| OD | 25.00 | + | – | + | – | + | + | + |
+: present −: absent
FL: fresh young leaves
AD: young leaves dry wind
OD: young leaves dry oven
The antioxidant activity in all extracts was determined by DPPH free radical scavenger assay. The highest percentage inhibition was found in the FL extract, while the OD extracthad the lowest antioxidant activity (Table 3). It seems that the antioxidant activity has positive correlation with the total phenolic and flavonoid contens in fresh leaves, which is strengthened by the low IC50 value. Phenolic constituents, such as flavonoids, phenolic acids and tannins, were ubiquitous secondary metabolites in plants. They were known to have antioxidant activity and it was likely that the activity of these extracts was due to these compounds.17
Table 2: Quantitative phytochemical analysis
| Exract | Total Phenolic Content (mg GAE/g extract) | Total Flavonoid content (mg QE/g extract) |
| FL | 25.35 ± 1.06 | 28.71 ± 2.07 |
| AD | 21.21 ± 1.29 | 20.04 ± 2.61 |
| OD | 7.54 ± 2.54 | 8.62 ± 3.43 |
Polyphenolic compounds in plants were reported to exert various biological effects, including antioxidant activity and carbohydrate hydrolyzing enzyme, due to their ability to bind with protein18. Various in vitro assay showed that many plant polyphenols in fact possessed carbohydrate hydrolyzing enzyme inhibitory activities that contributed to lower postprandial hyperglycemia in the management of diabetes. In this study, the hypoglycemic potential of R. fraxinifolius leaves was evaluated by the α-glucosidase inhibition assay. The AD extract demonstrated high inhibitory activity on α-glucosidase (Table 3). The quality of the enzymatic inhibition in these extracts was determined by calculating IC50 value, with the low number indicating high enzymatic inhibition quality. The order of the R. fraxinifolius leaf extract starting from the highest inhibitory activity by IC50 values could be established as followed: air drying (8.86 μg/mL) > fresh (12.35 μg/mL)> oven drying (27.55 μg/mL); this suggested that extract might potentially be used as anti-diabetic remedy.
Table 3: Biological activities of extract
| Exract | DPPH (IC50 μg/mL) | α-GIs activity (IC50 μg/mL) | Antibacterial activity (mm) | ||
| E. coli | S. aureus | B. subtilis | |||
| FL | 45.51± 5.03 | 12.35 ± 1.48 | 11 | 12 | 12 |
| AD | 52.22 ± 2.88 | 8.86 ± 1.41 | 12 | 10 | 13 |
| OD | 144.43 ± 6.19 | 27.55 ± 2.23 | 10 | 9 | 9 |
| Quercetin | 14.17±4.72 | 6.04±2.14 | – | – | – |
| Streptomycin Sulfat | – | – | 23 | 20 | 27 |
The study of α-glucosidase inhibitory activity of Rubus leaf extract was rarely reported compared to that of the fruit of Rubus sp extract.19 However, this finding supported the previous report that aqueous extract of Rubus sp leaves showed possible anti-diabetic activity in rats, and daily administration of 5g/kg leaves of the infusion decreased 50% glucose-induced hyperglycemia in alloxan-diabetic rabbits.17
Rubus species are known to contain secondary metabolites which active against some common pathogenic bacteria. Therefore, in this study, the extract was investigated on antibacterial activity against E. coli and S. aureus. The result of our current research demonstrated that all leaf extracts of R. fraxinifolius were moderate against Gram-positive and Gram-negative bacteria (Table 3) when compared to standard. Phenolic compounds such as flavone, quercetin, and naringenin potentially contributed to the antibacterial activities against S.aureus, B.subtilis, and E. coli.7 Our result showed that fresh and dried leaves had no noticiable difference on antibacterial activities. It might be that other active compounds, besides polyphenolic, contributed to antibacterial activity. This study was only a preliminary attempt to assess drying effect on bioactivity potential of R. fraxinifolius leaf extract. Hence, further detailed bioassay would need to be applied for assessing the antibacterial activity.
Profiling of LCMS-MS was conducted to identify bioactive compounds present in the R. fraxinifolius leaf extract (Figure 2). The chromatogram of methanol extract showed that oven drying could cause more degradation of several compounds in leaf extract than in both dried and fresh leaves. As shown in table 4, Luteolin-7-O-glucoronide, an active antioxidant flavonoid glycoside, was detected in fresh and air-dried extracts, while it was not found in the oven-dried leaf extract. There was no dry extract at the oven (Table 4).
 |
Figure 2: LCMS-MS chromatogram of the chemical constituents of Rubus leaf extract. (i) fresh leaves (FL), (ii) air dried (AD), and (iii) oven dried (OD) of young leaves Click here to View Figure |
Based on the results above, air drying could be chosen as a suitable technique for R. fraxinifolius leaf extract because it could still preserve the composition/content of the active compounds (antioxidants and lower hyperglycemia).
Table 4: Chemical composition of fresh and dried leaf extract
| Sample | Componentname | Observed m/z | Observed RT (min) |
| FL | Abrusoside A | 647.4794 | 5.63 |
| Epianhydrobelachinal | 469.3307 | 5.83 | |
| Epianhydrobelachinal | 469.3304 | 6.33 | |
| Luteolin-7-O-glucoronide | 463.0865 | 5.39 | |
| Poricoric acid B | 485.3255 | 7.12 | |
| AD | Abrusoside A | 647.4794 | 5.63 |
| Epianhydrobelachinal | 469.3307 | 5.83 | |
| Lucialdehyde B | 453.3353 | 9.32 | |
| Luteolin-7-O-glucoronide | 463.0866 | 5.39 | |
| Poricoric acid B | 485.3255 | 7.12 | |
| OD | Ephedradine C | 537.3033 | 9.56 |
| Epianhydrobelachinal | 469.3305 | 8.34 | |
| Epianhydrobelachinal | 469.3308 | 7.63 | |
| Epianhydrobelachinal | 469.3305 | 8.05 | |
| Lucialdehyde B | 453.3353 | 9.31 | |
| Poricoric acid B | 485.3255 | 7.10 |
Conclusion
Preliminary chemical examination indicated the presence of polyphenols and flavonoids, which might be responsible for antioxidant and antidiabetic (α-glucosidase inhibitory) activities. The presence of the phytochemical compounds in R. fraxinifolius leaf extract indicated that fresh or dried leaves of Rubus have medicinal potential and might function as antioxidant, anti-hyperglycemic, and anti-microbial. However, oven dried leaves had the least biological activities, implying that oven drying might not be an efficient method for drying rubus leaves. Further studies on the isolation of active constituent(s) along with further in vitro studies, need to be investigated in detail to explore the pharmaceutical and neutraceutical use of R. fraxinifolius leaf extract.
Acknowledgements
We would like to thank Mega Wijayanti, S.Si for her technical assistant in LCMS-MS analysis and Clarisa for manuscript editing.
Funding
This study was supported by National Innovation System Research Incentive Program (InSINas) 2018 from Ministry of Research, Technology and Higher Education (016/P/RPL-LIPI/INSINAS-1/III/2018).
Conflict of Interest
We have no conflict of interest to declare.
References
- Kalkman C. Rosaceae. Flora Malesiana ser. I. Leiden University. Leiden 1993;11(2): 227-351.
- Surya M.I. Keanekaragaman dan Potensi Rubus spp. Koleksi Kebun Raya Cibodas. Warta Kebun Raya. 2009; 9 (1): 21-26.
- Surya M.I., Suhartati S., Ismaini L., Lusini D., Anggraeni S., Normasiwi N., Asni and Sidiq M.A.B. Fruits nutrients content of five species of wild raspberries (Rubus spp.) from Indonesian Mountain’s Forest. Journal Tropical of Life Sciences.2018; 8 (1): 75-80.
CrossRef - Desmiaty Y., Elya B., Saputri F.C., Hanafi M., Prastiwi R. Antioxidant Activity of Rubus fraxinifolius Poir. and Rubus rosifolius J. Sm. Leaves. J Young Pharm. 2018; 10(2): Suppl: s93-s96
CrossRef - Riaz M., Ahmad M., Rahman N. Antimicrobial screening of fruit, leaves, root and steam of Rubus fructicosus L. J. Med. Plants Res. 2011; 5:5920-5924.
- Mediani A., Abas F., Tan C.P., Khatib A. Effects of Different Drying Methods and Storage Time on Free Radical Scavenging Activity and Total Phenolic Content of Cosmos caudatus. Antioxidants. 2014. 7;3(2):358-70. doi: 10.3390/antiox3020358.
CrossRef - Abu Bakar M.H., Ismail N.A., Azizul I. and Ling A.L.M. Phytochemical Composition and Biological Activities of Selected Wild Berries (Rubusmoluccanus L., R. fraxinifoliusPoir., and R. alpestris Blume). Evid Based Complement Alternat Med 2016; Article ID 2482930, 10 pages.
CrossRef - Shamsudin, N.A., Matawali, A., Gansau, J.A. Comparison of Antioxidant Activity and Phytochemical Content of Borneo Wild Berry, Rubus fraxinifolius (Rogimot), Transactions on Science and Technology. 2019; 6 (1): 36 – 41.
- Harborne J.B. Phytochemical methods: A guide to modern techniques of plant analysis, New York, Chapman and Hall, 3, 1998, 1-150.
- Yadav R.N.S. and Agarwala M. Phytochemical analysis of some medicinal plants, J. Phytol. 2011, 3(12): 10-14.
- Singleton V.L., Orthofehr, R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrate and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999; (299): 152-178.
CrossRef - Dewi, R.T and Maryani F. Antioxidant and α-Glucosidase Inhibitory Compounds of Centella Asiatica“, Procedia Chemistry. 2015; (17): 147 – 152.
CrossRef - Chang C.C., Yang M.H., Wen H.M., Chernn J.C., Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. Journal of Food and Drug Analysis. 2002;178-182.
- Valgas C., Souza S. M., Smânia E.F.A., Smania-Jr A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbial. 2007; 38:369-380.
CrossRef - Dewi R.T., Fitria I., Minarti, Sundowo A., et al. Screening of Potential Filamentous Fungi Aspergillus sp for Biotransformation of Quercetin. AIP Conference Proceedings 2024; (1):020017.
- Bernard D., Kwabena A.I., Osei O.D., Daniel G.A., Elom S. A., Sandra A. The Effect of Different Drying Methods on the Phytochemicals and Radical Scavenging Activity of Ceylon Cinnamon (Cinnamomum zeylanicum) Plant Parts. European Journal of Medicinal Plants 2014; 4(11): 1324-1335.
CrossRef - Rebaya A., Belghith A.I., Leddet V.M., Oliver E., Cherif J.K., Ayadi M.T. Total Flavonoid, Tannin Content, and Antioxidant Capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2014; 01: 052-057.
- Mai T.T, Thu N.N., Tien P.G., Chuyen N.V. Alpha-glucosidase inhibitory and antioxidant activities of Vietnam edible plants and their relationships with polyphenol contents. J Nutr. Sci. Vitaminol. 2007; 53:267-276.
CrossRef - Zia Ul-Haq M., Riaz M., De Feco V., Jaafar H.Z.E., Moga M. Rubus Fruticosus L: constituents, biological activities and health related uses. Molecules. 2014; 19: 10998-11029.
CrossRef


