Introduction
Gerbera is a genus of ornamental plants from the Asteraceae family. It has roughly 30 species in the wild, extending to South America, Africa and tropical Asia.1-2 The inflorescence of Gerbera hybrida are composed of three different types of flowers (ray, trans, disc) that are tightly packed into a condensed, radially organized capitulum.3 Most of the present commercially cultivated varieties originate from the artificial crossing progenies of G. jamesonii and G. viridifolia, both are South African species since natural hybrids of the two species have not been found.2
Gerbera is one of the most important commercially grown flower crops in the world. Gerbera is popular owing to its bright and vivid petal color, which is available in different shades and hues, and a large flower size. It has multiple uses in flower arrangements, bouquets, and dry flower crafts.4-5
However, they often suffer from short vase life.6 Addition of chemical preservatives to the holding solution is recommended to prolong the vase life of cut flowers. All holding solutions usually contain two ingredients namely, sugar and germicides. The sugars provide a respiratory substrate, while the germicides control bacterial growth and prevent plugging of the conducting tissues. Therefore, the techniques of prolonging the vase life of flowers will be a great asset to the growers and users.4 Different preservative mixtures are used for vase life prolongation of gerbera including nano silver6 and silver nitrate + sucrose.4 SA could increase peroxidase activity, raise fresh flower weight, enlarge flower diameter, and improve water balance of cut gerbera flowers .7 The positive effect of SA on fresh keeping of cut gerbera is confirmed by several reports.8-11 In this study, we aimed to find out the interaction effect among SA, 8-HQS, and sucrose on vase life of gerbera cut flowers.
Materials and Methods
Cut gerbera flowers were obtained from a local commercial greenhouse, and transported with proper covers immediately to Laboratory.
Solutions were freshly prepared at the start of experiments. Stems were recut to 40-50cm length. The study was arranged in a factorial test with complete randomized design with three replications. Each replication consisted of three cut flowers. Three levels of SA (0, 100 and 150 mg L -1), two levels of sucrose (0 and 3% w/v), and two levels of 8-HQS (0 and 200 mg L -1) were applied (total of 12 treatments). After recording the fresh weight, each flower was placed in a bottle containing 400 ml preservative solutions. The flowers were held at ambient temperature (22 ± 2 ℃). Except vase life all measurements including flower diameter and stem curvature were made at the 10th day of the experiment.
Vase life was determined as the number of days to wilting of flowers. The flowers were checked daily for signs of deterioration. All Flowers were weighed at the beginning and the 7th day of the experiment. The flowers were dried in the oven and the dry weight was calculated. Quality scoring was made based on the visual quality by grouping of replications to as much as possible distinct groups and then scoring them. The solution uptake was calculated by subtracting the mean volume of water evaporated from three-control bottles without cut-flowers, from the water decreased in bottles containing flowers during experimental course.
Analysis of variance was performed on the collected data using the general linear model (GLM) procedure of the SPSS software (Version 16, IBM Inc.). The mean separation was conducted by Duncan analysis in the same software (p≤ 0.05). For the data which were gathered once a day until 10th day of the experiment (wet weight, flower diameter and stem diameter), the GLM-repeated-measures module was applied for factorial analysis and related statistics is presented.
Results and Discussion
Duncan analysis confirmed that SA increased vase life and reduced stem curvature at 100 mg L-1 compared to 0 level. On the other hand, 150 mg L-1 of SA increased mean absorbed water per flower significantly compared to both 100 and 0 mg L-1 levels. 8-HQS increased vase life, dry weight, wet weight, flower diameter, solution uptake, and quality score. 8-HQS also decreased stem curvature from 59 to 16 degrees. Sucrose decreased vase life, flower diameter, and quality score, and increased dry weight (Table 1, F-test probabilities section).
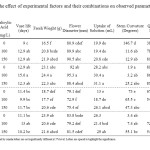 |
Table 1: The effect of experimental factors and their combinations on observed parameters in gerbera Click here to View table |
The mean comparison analysis revealed that the flower diameter showed the same exact pattern as was observed with vase life. The treatment containing just 8-HQS 200 mg L-1 had flower diameter of 92 mm which was not significantly different from that of the combination of SA 100 mg L-1 + 8-HQS 200 mg L-1 (95.3 mm). Both of these treatments were significantly different from that of control (80.9 mm). This is in agreement with the reports regarding the correlation between higher flower diameter and vase life of cut gerbera (12-13).
Any sign of “stem break” or “bent neck” which is reported as a common problem of cut gerbera (14) was not observed at all which might be related to nutrition state specially calcium content of cut gerbera. Instead, all treatments showed significantly lower stem curvature than the control treatment. The least curvature was recorded in 8-HQS 200 mg L-1 followed by SA 100 mg L-1 + 8-HQS 200 mg L-1 and 8-HQS 200 mg L-1 + sucrose 30 g L-1 , respectively. All of the combinations not containing sucrose showed higher quality scores than those containing sugar and control. Considering sucrose effects on other parameters we could conclude that at least in combination with SA and 8-HQS, no positive effect by sucrose was observed.
The following treatments had higher solution uptake than control treatment and were classified in the same category; SA 150 mg L-1 + 8-HQS 200 mg L-1,SA 100 mg L-1 + 8-HQS 200 mg L-1, SA 150 mg L-1 + Sucrose 30 g L-1 + 8-HQS 200 mg L-1 and 8-HQS 200 mg L-1. Presence of SA in ¾ of treatments with the best performance regarding uptake of preservative solution agrees with previous results on the positive effects of SA on uptake of preservative solution.7,11
The significant decrease in dry weight loss, both by sucrose and 8-HQS was noticed. While this could be considered normal for sucrose as a carbohydrate, the increase in dry weight by 8-HQS suggests that in addition to control of microbial proliferation in preservative solution, it could have acted as a carbon source for cut flower as well.
The treatment containing just 8-HQS (200 mg L-1) had vase life of 12.9 days, which was not significantly different from the combination of SA 100 mg L-1 + 8-HQS 200 mg L-1, which resulted the longest vase life of 15.6 days. Both of these treatments were significantly different from that of control (9 days). Both of these treatments were unique in sharing the best statistical group in all other measured traits including; stem curvature, flower diameter, solution uptake, dry and wet weight.
As we see in Table 1, most treatments showed significantly more vase life compared to control. The treatment containing just 200 mg/L 8-HQS and 100 mg/L SA had the longest vase life of 15.6 days compared with the same combination plus 30 g/L Sucrose with 13 days, which stood on the next step. This indicates that this combination without Sucrose has yielded better in term of vase life. Although the Sucrose containing combination still is in the same statistical group with the superior treatment, when considering other traits the difference becomes apparent. While the superior treatment is in the best statistical group in all other recorded traits, the later fails to do so in most important traits including fresh weight, flower diameter and absorbed preservative solution, which are indicators of water balance in cut flowers. When considering the dry weight we see that the treatment with sucrose has a higher dry weight, which shows that sucrose had helped the cut flower to maintain its carbohydrate level while it seems that the cut flower had performed better when dependent on its own carbohydrate storage. Sucrose was not considered beneficial in our study at all compared with combinations of SA and 8-HQS. Fan Mei-Hua10 suggested that SA could take place of 8-hydroxyquinoline as shelf-life-enhancing liquid agents in combination with BA and sucrose, but our results suggest that SA, alone or in combination with sucrose, could not surpass 8-HQS concerning positive effect on vase life. We conclude that combining SA with8-HQS based preservative mixtures could improve its effect further.
References
- Bremer K. Asteraceae: cladistics and classification: Timber Press: Portland, Oregon; 1994.
- Hansen HV. A taxonomic revision of the genus Perdicium (Compositae Mutisieae). Nordic journal of botany1985;5(6):543-6.
CrossRef - Kotilainen M, Elomaa P, Uimari A, Albert VA, Yu D, Teeri TH. GRCD1, an AGL2-like MADS box gene, participates in the C function during stamen development in Gerbera hybrida. The Plant Cell Online2000;12(10):1893.
CrossRef - Nair SA, Singh V, Sharma T. Effect of chemical preservatives on enhancing vase-life of gerbera flowers. Journal of Tropical Agriculture2003;41:56-8.
- Patel T, Singh A, editors. Effect of Different Modified Atmosphere Packaging (MAP) Films and Cold Storage Temperatures (5, 10 and 15 C) on Keeping Quality of Gerbera (Gerbera jamesonii) Flowers2008.
- Liu J, He S, Zhang Z, Cao J, Lv P, Cheng G, Joyce DC. Nano-silver pulse treatments inhibit stem-end bacteria on cut gerbera cv. Ruikou flowers. Postharvest Biology and Technology2009;54(1):59-62.
CrossRef - Jing H, Luo H, Li J. Physiological actions of preservatives containing salicylic acid and benziond acid on cut Gerbera flower [J]. Journal of Central China Normal University (Natural Sciences)2004;1.
- Jian-bo B. Comparative Study of the Effects of Salicylic Acid and Nitricacid Silver on Gerbera jamesonii Bolus. Journal of Anhui Agricultural Sciences2009;33.
- Li-mei D. Fresh-keeping Effect of a New Fresh-keeping Agent with Salicylic Acid on Cutting Flowers of Gerbera jamesonii [J]. Guizhou Agricultural Sciences2010;6.
- 10. Mei-hua F, Jian-xin W, Ge S, LI-na S, Ruo-fan L. Salicylic Acid and 6-BA Effects in Shelf-life Improvement of Gerbera jamesonii Cut Flowers. Northern Horticulture,(8)2008:117–20.
- Yuping Z. Effects of Salicylic Acid on Fresh Keeping of Cut Gerbera Jamesonii Flower. Anhui Agricultural Science Bulletin2009.
- Ansari S, Branch K, Hadavi E, Salehi M, Moradi P, Branch S. Application of Microorganisms Compared with Nanopar-ticles of Silver, Humic Acid and Gibberellic Acid on Vase Life of Cut Gerbera Goodtimming. Journal of Ornamental and Horticultural Plants2011;1(1):27-33.
- ZHANG X-p, QI X-y, WANG X-j, ZHOU Y-l. Effect of Organic Acid on the Preservation of Cut Chrysanthemum. Heilongjiang Agricultural Sciences2011;5:023.
- Abdel-Kader H, Rogers MN, editors. Postharvest treatment of Gerbera jamesonii. III International Symposium on Postharvest Physiology of Ornamentals 181; 1985.
