Introduction
Capsicum annuum L. (chili pepper) is one of the horticultural plants that have high economic value in Indonesia. It mainly used daily as a food ingredient, pickle or as materials for a food industry. Besides that, it is also known as one component of herbal medicine.1 In Indonesia, the productivity of chili pepper shows high fluctuation. It was reported that in 2015, the productivity of chili pepper was 1.045.182 ton, while in 2016 the productivity increased sharply to 10.205.694 ton.2 Several factors contributed to the productivity of chili pepper such as high pest and disease, climate change, as well as the used of a low quality of chili pepper seed cultivars.
Breeding of chili pepper is important to improve its characteristics and to increase genetic variability. High genetic variation provides choices for high quality of chili pepper. Plant breeding can be done through conventional breeding using artificial crosses. Intraspecific and interspecific breeding generally used to obtain superior varieties.3 Modern plant breeding has also been developed which employs molecular technology and recombination of specific genes.4
Mutation breeding is one method that complemented conventional breeding as a tool to increase diversity and generate raw material which in turn through selection process can produce plants with better quality.5 Induced mutation is generally conducted using gamma radiation as physical mutagen or using chemical mutagens as well as a combination of physical and chemical mutagens.6 For example, treatment using gamma ray has the potential to increase resistance to Begomovirus in chili.7 Several common chemical mutagens used in plant breeding are colchicine and oryzalin to double chromosome number. These two mutagens are anti-microtubule that inhibits generation of microtubule and induce development of polyploidy in plant.8 Other chemical mutagen highly used in plant mutation breeding is Ethyl methanesulfonate (EMS) and sodium azide (NaN3), which induces point mutation. Ethyl methanesulfonate was used to develop tomato with high resistance to Orobanche ramosa L,9 to increase the yield of Vigna radiata.10 In C. frustescens, sodium azide was used to alter growth traits such as increasing number of stems.11
Previous study reported that more than 80% new plant mutant registered at the database of the International Atomic Energy Association (IAEA), resulted from induced mutation using chemical mutagens that work as alkylating agens.12 Ethyl methanesulfonate (CH3SO2OC2H5) is an alkylating agent which alkylates guanine into O6-ethylguanin and changes the pairing of Guanine-Cytosine to become O6-ethylguanin-Tymin.13 It works efficiently and potentially to an induced mutation in plant.14
This study aimed to evaluate EMS concentrations that induce mutation in chili pepper and to analyze the morphological variations induced by EMS at M2 generation. In the M2 generation, the mutation will segregate to create homozygotes for recessive or dominant alleles.15 At M2 generation, the alleles will segregate into homozygotes recessive or homozygotes dominant and at this generation, the most effective method to observe phenotypic mutation is using visual screening.16 Through induced mutation, a variation of chili pepper can be obtained and it is expected that the variation can be useful to overcome the problem of chili pepper cultivation in the future.
Materials and Methods
Plant Materials
Seeds of C. annuum ‘Hot Pepper Smart’ were purchased from a local nursery in Denpasar, Bali, Indonesia. The planting site was at filed station facility of Faculty of Agriculture, Udayana University, Denpasar, Bali, Indonesia.
Mutagenesis, Planting and Data Collection
The C. annuum seeds (M0) were pre-soaked in water for 6 hours and treated with 0.5%, 0.75%, and 1% EMS in phosphate buffer pH 7.0 for 6 hours. As the control, seeds were soaked in phosphate buffer pH 7.0. The seeds were then washed in running water for 5 hours and they germinated in the soil in seedling trays. The three weeks seedlings were planted in the field to form M1 population.
Seeds from M1 plants were bulked for each treatment and sown as the M2 generation. Three weeks after sowing, seedlings were transferred into the polybag with topsoil, organic compost and rice husk (2:1:1) as growing media. One seedling was planted into one polybag. The polybags were arranged into progeny raw with 50 cm × 50 cm spaces between polybags. The number of seedling planted were 90 for control, 95 for 0.5% EMS, 98 for 0.75% and 104 for 1% EMS.
Plants were watered once a day and fertilized every month start from 4 WAT (week after transfer). Fertilizer was made by dissolving 2 g NPK (15:15:15) in 1 L water, and each plant was fertilized with 250 ml fertilizer.17 Observations were done on percentage of seedling emergence in seedling trays, plant height and percentage of plant survival at maturity. The number and types of morphological changes in M2 plants were observed visually.
Results and Discussion
Seedling emergence in M2 was recorded up to 4 weeks after sowing and the maximum percentage of seedling emergence occurred at control seeds, but it was only slightly higher than that of 0.5% EMS seeds. The seedling emergence of M2 was low in all treatments including control. The lowest percentage of seedling emergent was 46% at 0.75% EMS (Table 1).
The effect of EMS to plant variation and growth was influenced by the amount of mutagen uptake or part of embryo affected by EMS.18 High concentration of EMS inhibited physiological process for seed germination including inhibition of catalase and lipase activity, hormone imbalance and mitosis inhibition which lead to poor growth.19 The seedling emergence was low at M2 plant population, while the M1 population had higher seed emergence. The ability of seed affected by EMS to germinate depends on C. annuum cultivars and the experimental condition used.20 Previous study also observed a further reduction in germination at M2 generation where mostly the germination was in the range of 41% to 60%.21 Other study showed that low germination was observed at M1 generation.20
Table 1: Percentage of seedling emergence and plant survival at maturity.
| M2 seed |
M1 seedling emergence (%) |
M2 seedling emergence (%) |
M2 plant survival at maturity (%) |
| Control |
90.7 |
54.2 |
87.7 |
| 0.5% EMS |
88 |
52.7 |
69.5 |
| 0.75% EMS |
76 |
46 |
70.4 |
| 1% EMS |
72 |
50 |
78.8 |
The range of plant height at maturity at control plants was 45.6 cm to 67.7 cm. The height of tall plants was 85.2 cm (1-L1-11) and 94.6 cm (1-L4-8). Plant with 80 cm to 100 cm height was classified as long plant.21 In this study, one M2 plant with two main stems was observed. This type of mutation resulted from treatment using 1% EMS. Each stem was able to developed fruit. Another type of M2 recorded was the plant with small size of leaf and yellowish leaf colour (1-L4-4).
The M2 plants from 0.5% EMS treatment had the lowest survival. The highest survival rate was in control plant, followed by the M2 plant from 1% EMS treatment (Table 1). The plant survivals at maturity were lower in M2 plants than in control plants. Similar results in M2 of C. annuum under EMS treatments were reported earlier.21,22 Reduced plant survival may due to the occurrence of random point mutations and chromosomal breaks that lead to lethal effects.23 The chromosomal injury caused by EMS was observed in winged bean which affected plant survival.24 In the M1 plant of C. annuum treated with 0.8% EMS and 1 EMS, high level of abnormal chromosome configuration in meiosis was observed which reduced plant vigour.22
The morphological changes at M2 plants were recorded. The highest percentage of mutation was obtained in 1% EMS treatment. A summary of individual changes is shown in Table 2. Figure 1 and 2 show the different of M2 plant morphology compared to control plants. A plant is categorized as a dwarf if the height of the plant is less than 20 cm.21 In this study, the height of the two dwarf plants identified was 16 cm (1-L3-15) and 17.5 cm (1-L2-8). Both of the dwarf plants were able to develop fruit however the plants did not survive to the stage of fruit maturation. Figure 3 shows differences between the mutant plant with pale green leaf colour and control plant.
Table 2: Types of morphological mutation.
| Planta | Morphological mutation |
Percentageb (%) |
Survival |
| 1-L1-11, 1-L4-8 | Tall |
1.92 |
Survived |
| 1-L4-4 | Small and pale green leaf colour |
0.96 |
Survived |
| 1-L1-8, 1-L4-8 | Some flowers have seven petals |
1.92 |
Survived |
| 1-L2-8, 1-L3-15 | Dwarf |
1.92 |
Developed fruits but did not survive to harvesting time |
| 1-L4-6 | Tall, two main stems |
0.96 |
Survived |
|
Total 1% EMS |
7.68 |
||
| 0.75-L3-9 | Short and many branches |
1.02 |
Survived |
|
Total 0.75% EMS |
1.02 |
||
| 0.5-L2-11 | Pale green leaf colour |
1.05 |
Survive |
|
Total 0.5% EMS |
1.05 |
a1, 0.75, 0.5: EMS concentration; L1…L4: planting position (lane); 1…15: plant number
bNumber of mutants divided by number of seedlings planted in each treatment
 |
Figure 1A): Mutant short plants with many branches (left), mutant dwarf plant with less branch and small leaf (center) and control plant/wild-type (right). From left: 0.75-L3-9, 1-L2-8, control. B) Performance of mutant tall plant (left), mutant small plant (center) and control plant/wild-type (right). From left: 1-L1-11, 1-L2-8, Control Click here to View figure |
 |
Figure 2A): Mutant tall plant with two main stems (1-L4-6). B). Mutant plant with small size and pale green colour leaves (1-L4-4) (left) and control plant/wild-type (right) Click here to View figure |
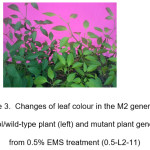 |
Figure 3: Changes of leaf colour in the M2 generation. Control/wild-type plant (left) and mutant plant generated from 0.5% EMS treatment (0.5-L2-11) |
Mutation is a random process, therefore the genome is damaged randomly in each cell in the treated seed in the M1 generation. Different cells of the same seed will contain different mutations.23 In this study, the EMS treatments resulted in abnormality such as dwarf plants which did not survive therefore the mutation has negative value. However, mutagenesis with EMS also resulted in tall plants and plants with two main stems that are useful in agriculture.
Dwarf mutants seem to be common mutant resulted from EMS treatments. Dwarf plants were previously observed at an induced mutation in C. annuum cv. Longhi using EMS at low concentrations ie. 0.01% for 6 hours, 0.1% for 3 hours and 0.1% for 6 hours.20 The 0.6% EMS for 12 hours also induced dwarf plant of C. annuum cv B12 from Capsicum Research Group from the College of Horticulture at Northwest A&F University, China, at M2 generation.21 Dwarf plant might have resulted from the inhibition of the elongation of epidermal cells.25 In addition, it has been known that dwarf mutant occurred due to reduced levels of gibberellic acid (GA).25,26 Treatment with EMS may lead to the damage of GA biosynthesis.21
Conclusions
The EMS treatments at concentrations of 0.5%, 0.75% and 1% resulted in various mutants at M2 of C. annuum, including tall plants, small plant with pale green leaf colour, plants with seven petals, dwarf plants, tall plant with two main stems, short plant with many branches and plant with pale green leaf colour. Based on the percentage of mutants, 1% EMS treatment was more effective in generating mutant of M2 generation as compared to 0.5% EMS and 0.75% EMS.
Acknowledgements
This study was funded by Directorate General of Higher Education of Republic Indonesia through Hibah Bersaing Research Grant Scheme.
References
- Heyne, K. Tumbuhan berguna Indonesia Jilid III. Yayasan Sarana Wana Jaya, Jakarta. 1987.
- BPS, 2017. Laporan ringkas studi cabai. Laporan bulanan data sosial ekonomi. Badan Pusat Statistik, Jakarta. 2017.
- Stoskopf, N. C. D., Tomes D. T. and Christie B. R. Plant breeding, theory and practice. Westview Press, Oxford. 1983.
- Breseghello, F. and Coelho, A. S. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013;61(35):8277-8286.
CrossRef - Adamu, A. K., Clung, S. S. and Abubakar, S. Effects of ionizing radiation (gamma-rays) on tomato (Lycopersicon esculentum). Nigeria J. Exp. Appl. Biol. 004;5:185-193.
- Mondal, S., Badigannavar, A.M., Kale, D.M. and Murty, G.S.S. Induction of genetic variability in a disease-resistant groundnut breeding line. BARC Newsletter. 2007;258:237-247.
- Gaswanto, R., Syukur, M., Purwoko, B. S. and Hidayat, S. H. Induced mutation by gamma rays irradiation to increase chilli resistance to begomovirus. Agrivita. 2016;38(1):24-32.
CrossRef - Liu, X.Z., Lin,H., Mo, X. Y., Long, T. and Zhang, H. Y. Genetic variation in colchicine-treated regenerated plants of Eucalyptus globulus Labill. J. Genet. 2009;88: 345-348.
CrossRef - Kostov, K., Batchvarova, R. and Slavov, S. Application of chemical mutagenesis to increase the resistance of tomato to Orobanche ramosa L. Bulgarian J. Agric. Sci. 2007;13:505-513.
- Khan, S. and Wani, M. R. Genetic variability studies for seed yield and its components in mungbean (Vigna radiata (L.) Wilczek). Thai. J. Agric. Sci. 2006;39(1–2):83–88.
- Saraswati, I. G. A. E., Pharmawati, M. and Junitha, I. K. Morphological characters of chilli pepper (Capsicum frutescens L.) as influenced by sodium azida at generative stage of M1 generation. J. Biol. 2012;16(1):23-26.
- Oladosu, Y., Rafii, M. Y., Abdullah, N., Hussin, G., Ramli, A., Rahim, H. A., Miah G. and Usman, M. Principle and application of plant mutagenesis in crop improvement: a review. Biotechnol. Biotechnol. Equip. 2016;30(1):1-16.
CrossRef - Henikoff, S. and Comai, L. Single-nucleotide mutations for plant functional genomics. Annu. Rev. Plant Biol. 2003;54:375-401.
CrossRef - Natarajan, A. T. Chemical mutagenesis: from plants to human. Curr. Sci. India. 2005;89(2):312-316.
- Page, D. R. and Grossniklaus, U. The art and design of genetic screens: Arabidopsis thaliana.Nat. Rev. Genet. 2002;3:124–136.
CrossRef - Østergaard, L. and Yanofsky, M. F. Establishing gene function by mutagenesis in Arabidopsis thaliana. Plant J. 2004;39:682–696.
CrossRef - Darmawan, E. Kajian daya hasil tiga varietas cabai merah besar (Capsicum annuum L.) akibat pemberian jenis pupuk. Skripsi. Program Studi Agronomi, Fakultas Pertanian Universitas Jember. 2006.
- Boranayaka, M. B., Kambegowda, R., Nandini, B., Satish, R.G., Santoskumar, B. and Pujer. Influence of gamma rays and ethyle methane sulphonate on germination and seedling survival in sesame (Sesamum indicum L.). Int. J. Pl.Sci. 2010;5:655–59.
- Joshi, N., Ravindran, A. and Mahajan, V. Investigation on chemical mutagen sensitivity in onion. Int. J. Bot. 2011;7:243-248.
CrossRef - Jabeen, N. and Mirza, B. Ethyl methane sulfonate induces morphological mutations in Capsicum annuum. Int. J. Agric. Biol. 2004;6:340-345.
- Arisha, M. H., Shah, S. N. M., Gong, Z.-H., Jing, H., Li, C., and Zhang, H.-X. Ethyl methane sulfonate induced mutations in M2 generation and physiological variations in M1 generation of peppers (Capsicum annuum L.). Front. Plant Sci. 2015;6:399; http://doi.org/10.3389/fpls.2015.00399.
CrossRef - Dhamayanthi, K. P. M. and. Reddy, V. R. K. Cytogenetic effect o gamma rays and ethyl methane sulphonate in chilli pepper (Capsicum annuum L.) Cytologia. 2000;6:129-133.
CrossRef - Greene, E. A., Codomo, C. A., Taylor, N. E., Henikoff, J. G., Till, B. J., Reynolds, S. H., Enns, L. C., Burtner, C., Johnson, J. E., Odden, A. R., Comai, L. and Henikoff, S. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics. 2003;164:731–740.
- Sonavane, A. S. Effect of EMS and SA on survival of plants at maturity in M1 generation of Psophocarpus tetragonolobus (L).DC. Int. J. Adv. Res. 2017;5(2):2637-2639.
CrossRef - Fridborg I., Kuusk, S., Moritz, T. and Sundberg, E. The Arabidopsis dwarf mutant shi exhibits reduced gibberellin responses conferred by overexpression of a new putative zinc finger protein. Plant Cell. 1999;11: 1019–1031.
CrossRef - Sikora, P., Chawade, A., Larsson, M., Olsson, J. and Olsson, O. Mutagenesis as a tool in plant genetics, functional genomics, and breeding. Int. J. Plant Genomics. 2011;314829;doi: 10.155/2011/314829.


