Introduction
A member from the Meliaceae family, neem (Azadirachta indica) is frequently described as “Indian lilac” or “Margosa.” Insecticides, pesticides, and agrochemicals can be found naturally in neem.1 Since ancient times, Indians have been aware of neem’s medicinal benefits. Ayurvedic, homoeopathic, and unani medicine have all made substantial use of neem. Numerous pharmacological properties of neem leaves include anti-hyperglycemia, anti-inflammatory, anti-malaria, anti-ulcer, antiviral, antibacterial, antifungal, antioxidant, anti-mutagenic, anti-carcinogenic and immune modulatory. Neem is an integral part of Indian rural medicine.1,2
Even after having antimicrobial potential, neem is susceptible to the infections and diseases caused by microorganisms. Many bacterial and fungal pathogens incite disease on neem.3 Die-back disease caused by Phomopsis azadirachtae Sateesh, Bhat, and Devaki3,4 is presently a major harmful disease of neem. The disease results in severe decrease of evergreen canopy and also nearly 100% loss in fruit production is observed. This severely affects the health of the plant as well as seed availability.5 P. azadirachtae, is disseminating at a very fast rate3 and thus proper management is the need of the hour.
P. azadirachtae is reported to be inhibited by a few chemicals.6 Since fungicides are known to be biohazardous, alternate methods (biofungicides) for managing plant diseases must be developed.7 Microbial communities in natural ecosystems, especially in soil, are known to inhibit the growth of pathogenic microbes by their antagonistic properties.8
Andrographis paniculata, a medicinal plant and annual herbaceous plant, is a member of the Acanthaceae family. It is extensively grown in China, India, southern Asia, and some regions of Europe. Because of the different pharmacological effects it has, including antimicrobial activities, A. paniculata has been used for generations as a medicine.9 Review of literature indicates that majority of research works are concentrated on antimicrobial activities of A. paniculata extracts.9,10 Work on screening of A. paniculata rhizosphere bacteria for antifungal activity is nil. Owing to this the present investigations were carried out with the objectives of isolation of bacteria from A. paniculata rhizosphere and evaluation of their antagonistic potential against P. azadirachtae.
Materials and Methods
Isolation of rhizobacteria
Samples of Andrographis paniculata rhizosphere soil were gathered. 1.0 g soil sample was subjected to serial dilution using sterile physiological saline up to 10-9 dilution. Using a sterile spreader, inoculum of 0.1 ml from 10-7, 10-8, 10-9 dilutions was spread on solidified nutrient agar (NA) plates aseptically. Subsequently, the plates were maintained at 37°C over 24 – 48 hours. Following incubation, streaking was used to pure-culture the distinct-looking cultures onto nutrient agar slants, which were then kept at 4oC until needed.
Screening of rhizobacteria by dual-culture method against Phomopsis azadirachtae
Sterile PDA (potato dextrose agar) medium was poured (20 ml) to sterile Petriplates and allowed to solidify. 5.0 mm mycelial disc of P. azadirachtae culture (7-days-old) was placed in middle of the plate, while bacterium was streak inoculated above the fungal disc at a distance of 2.0 cm. All 10 bacterial isolates underwent equal process. The control group consisted of PDA plates that were just inoculated with the fungal disc. For 7-10 days, inoculated Petriplates were incubated at 30°C. After incubation, plates were examined for bacterial antifungal activity (a decline in fungal mycelial growth and formation of an inhibition zone between bacterial streak and fungal culture). Strains that demonstrated strongest suppression of fungal growth were chosen for additional research.
Characterization of rhizobacteria having substantial antagonism against Phomopsis azadirachtae
Apart from staining, biochemical assays, including IMViC, casein hydrolysis, gelatin hydrolysis, starch hydrolysis, triple-sugar iron agar, as well as catalase were carried out in compliance with standard procedures11 to identify the chosen bacterial strains.
Extraction of ethyl acetate fraction from the filtrates of bacterial cultures
100 ml nutrient broth taken in a 500 ml Erlenmeyer flask was inoculated with a loop of rhizobacteria culture. Each bacterium was inoculated into ten flasks. All of the flasks were maintained at 37°C for 72 hours. The cells were then separated and the supernatant was collected using centrifugation (9000xg, 10 min, 4ᵒC). This culture filtrate was filtered using 0.45 μm membrane filter (Sartorius, Gottingen, Germany), and diluted to 1.5 litres adding sterile distilled water. Employing a flash evaporator at 50°C, culture filtrates volume was condensed to 10 per cent of the original volume (i.e. from 1.5 litres to 150 ml). 150 ml of bacterial culture was made to have a pH of 3.6 using 1.0 N HCl in order to be extracted. The culture filtrates, using the same amount of ethyl acetate, were extracted three times, and each time the organic fraction was collected. A brownish, semi-solid crude extract was obtained after pooling as well as evaporating organic extracts around room temperature. It was then weighed and kept at 4oC.
Screening of ethyl acetate extracts for antifungal potential
A bacterial ethyl acetate fraction stock solution (1000 ppm) was made by dissolving the obtained fraction in sterilized distilled water with 1.0 mg/ml of Tween-20 (0.1%). 0.1% Tween-20 solution in sterile distilled water served as control.
The poison-food method was used to screen the antifungal activity of bacterial ethyl acetate fraction. In the initial screening, several concentrations, 2, 4, 6, 8, and 10 ppm, were obtained by adding appropriate portion of ethyl acetate fraction stock solution into 100 ml of sterile PDA medium in flasks, individually. Similarly concentrations of 20, 40, 50, 60, 80 and 100 ppm were prepared in further screening. PDA (100 ml) consisting either 10 ppm or 100 ppm of control solution was used as control. Twenty millilitres from every treated PDA were added onto separate Petri dishes of 9.0 mm diameter, left to set, and then inoculated with a 5.0 mm mycelial-agar disc of P. azadirachtae culture (7 days old) at the centre. Inoculated Petri dishes were then maintained at room temperature with 12-hour photoperiod for 10 days. Experiment was performed twice, with three replications each time. After incubation, each ethyl acetate fraction concentration’s effect on mycelial development was noted down in terms of colony diameter. To estimate fungitoxicity, the colony diameter in test plates was compared with that of the control plate. Pycnidial number was noted down after 15 days of incubation.
Results
Isolation and selection of rhizobacterial strains
The rhizosphere soil yielded ten distinct bacterial species. The bacterial species were named as isolates ARB1 to ARB10 (ARB Andrographis Rhizobacteria). Three bacterial strains (ARB1, ARB3, and ARB4) that showed the best inhibition activity (a decline in fungal mycelial growth and formation of an inhibition zone between bacterial streak and fungal culture) in the dual culture method (Fig. 1) were selected.
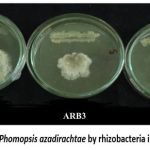 |
Figure 1: Inhibition of Phomopsis azadirachtae by rhizobacteria in dual culture method. |
Biochemical characterization of rhizobacteria
Table 1 lists the outcomes of the biochemical and staining tests. Based on the results of biochemical tests conducted it was not possible to precisely identify the bacteria using Bergey’s manual of determinative bacteriology. Few more biochemical tests and molecular characterization are required to identify the bacteria precisely.
Table 1: Biochemical characterization of rhizosphere bacteria
| Tests | APRB1 | APRB3 | APRB4 |
| Gram Staining | Gram positive | Gram-positive | Gram negative |
| Shape | cocci | cocci | cocci |
| Catalase test | Positive | Positive | Negative |
| Indole | Positive | Positive | Positive |
| Methyl Red | Positive | Negative | Positive |
| Citrate utilization | Positive | Negative | Positive |
| Triple sugar iron agar | AlS, AcB, Gp | AlS, AcB, Gp | AlS, AcB, Gp, H2S |
| Voges Proskauer | Negative | Positive | Negative |
| Starch hydrolysis | Positive | Positive | Positive |
| Casein hydrolysis | Negative | Negative | Negative |
| Gelatin hydrolysis | Positive | Positive | Positive |
AlS – alkaline slant, AcB – acidic butt and Gp – gas production
Extraction of ethyl acetate fraction from the filtrates of bacterial cultures
Table 2 illustrates the quantity of ethyl acetate fractions extracted from culture filtrates of three rhizobacteria.
Table 2: Amount of ethyl acetate fractions from culture filtrates of three rhizobacteria
| Bacteria | Ethyl acetate fractions (mg) |
| ARB1 | 79.2 |
| ARB3 | 83.7 |
| ARB4 | 70.2 |
Screening of ethyl acetate extracts for antifungal potential
In 2, 4, 6, 8, and 10 ppm concentrations of their ethyl acetate fraction, none of the three rhizobacteria (ARB1, ARB3, and ARB4) could totally prevent Phomopsis azadirachtae from growing. As a result, the experiment was conducted again with higher ethyl acetate fraction concentrations (20, 40, 50, 60, 80, and 100 ppm). Around 40 ppm concentration of their ethyl acetate component, all showed total suppression of the fungal mycelial development (Table 3). All bacteria exhibited similar level of pathogen growth control at 40 ppm. Pycnidia development was totally prevented at still lower concentrations – at 8 ppm by ARB1 and at 10 ppm by ARB3 and ARB4. With the increase in concentration of extract, growth of P. azadirachtae as well as pycnidia production decreased proportionately (Table 3). The results indicated substantial antifungal activity of all the three isolated rhizobacteria against the die-back pathogen P. azadirachtae.
Table 3: Effect of Ethyl acetate fraction of rhizobacteria at different concentrations against Phomopsis azadirachtae
| Concentrations | ARB1 | ARB3 | ARB4 | |||
| Diameter of the mycelialmat (mm) | Number of Pycnidia | Diameter of the mycelialmat (mm) | Number of Pycnidia | Diameter of the mycelialmat (mm) | Number of Pycnidia | |
| Control | 90.0 ± 0.93 | 195 ± 1.48 | 80.33 ± 0.95 | 187 ± 1.39 | 84.0 ± 0.68 | 239 ± 1.57 |
| 2 ppm | 79.0 ± 0.89 | 125 ± 1.34 | 80.0 ± 0.93 | 120 ± 1.48 | 81.0 ± 0.82 | 158 ± 1.88 |
| 4 ppm | 59.33 ± 0.88 | 55 ± 1.41 | 72.0 ± 0.73 | 56 ± 1.55 | 78.66 ± 0.99 | 97 ± 1.06 |
| 6 ppm | 36.66 ± 0.80 | 14 ± 0.86 | 40.66 ± 0.80 | 25 ± 1.29 | 45.66 ± 0.71 | 34 ± 1.34 |
| 8 ppm | 26.0 ± 0.86 | 0 | 35.33 ± 0.56 | 19 ± 1.0 | 27.0 ± 0.77 | 12 ± 0.93 |
| 10 ppm | 17.33 ± 0.49 | 0 | 23.33 ± 0.76 | 0 | 21.66 ± 0.76 | 0 |
| 20 ppm | 12.66 ± 0.71 | 0 | 12.66 ± 0.71 | 0 | 11.0 ± 0.63 | 0 |
| 40 ppm | 0 | 0 | 0 | 0 | 0 | 0 |
| 50 ppm | 0 | 0 | 0 | 0 | 0 | 0 |
| 60 ppm | 0 | 0 | 0 | 0 | 0 | 0 |
| 80 ppm | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 ppm | 0 | 0 | 0 | 0 | 0 | 0 |
Values are the average of two experiments, each with three replicates ± SE
Discussion
Globally phytopathogens cause a serious threat to plant health and productivity. Management of phytopathogens is still mainly attained by the application of chemical products. The natural environment is harmed by these pesticides because they increase environmental pollutants and produce residue build up. Biocontrol of phytopathogens using microorganisms has been found to minimize pollution and problems coupled with synthetic chemicals usage.12 Microbes in the rhizosphere region, through competition and different antagonistic mechanisms, exhibit significant antimicrobial effects in opposition to plant diseases.13
In this study, bacteria have been obtained from A. paniculata plant’s rhizosphere and first tested against P. azadirachtae by the dual culture method followed by poison-food method using the ethyl acetate extract of their culture filtrates. Three bacteria were isolated and named as ARB1, ARB3 and ARB4. All three bacteria showed significant inhibition of P. azadirachtae both in the dual culture method as well as in the poison-food method. Dual culturing is a regularly employed preliminary method to test the antagonistic potential of microbes. Many researchers have used this procedure to successfully isolate antifungal bacteria.14,15,16 The poisoned food method is the frequently used approach to study antifungal activity.17 Isolation and testing of rhizobacteria from other plants to control phytopathogenic fungi have been reported.15,16 Rhizosphere of medicinal plants is an excellent habitat for vast number of microorganisms and the rhizobacteria present here are known to serve variety of purposes including plant pathogen control.18
Microbes that inhibit phytopathogens are reported to produce various secondary metabolites. Examples are antibiotics and toxins, polyketides (PKs), ribosomal peptides (RPs), and volatile organic compounds (VOCs), & non-ribosomal peptides (NRPs)13, which provide antagonistic potential to microbes to inhibit phytopathogens. The ethyl acetate extracts of rhizosphere bacteria used in this study might be rich in such secondary metabolites leading to antagonistic activity, but precise understanding of this requires further research. To our knowledge, this is the first report of antifungal activity of A. paniculata rhizobacteria against fungal phytopathogens, particularly against P. azadirachtae.
Conclusion
This die-back disease is causing a serious problem to the growth of neem trees. This demands immediate attention for effective management. In the current investigation, the rhizobacteria of the A. paniculata plant have exhibited effective results against P. azadirachtae by completely suppressing the pathogen’s growth at low concentrations. Thus, this could provide an efficient, environment-friendly method of controlling P. azadirachtae if developed as a biopesticide or as an integrated disease control strategy.
Acknowledgement
The authors sincerely thank Science and Engineering Research Board (SERB), New Delhi and Department of Microbiology, Maharani’s Science College for Women, Mysuru for providing laboratory facility.
Funding Sources
Science and Engineering Research Board (SERB), New Delhi (SERB No. SB/FT/LS-416/2012; OYS scheme)
Conflict of Interest
The authors do not have any conflict of interest.
Data availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Author contributions
Sheethal Prabakar: Data collection, Analysis, Writing – original draft
Girish Krishna: Conceptualization, Methodology, Writing – Review and Editing
References
- Girish K., Shankara Bhat S. Neem – a Green Treasure. . EJBio. 2008a;4(3):102–111.
- Majib S., Modak S. Neem: Treasure of Natural Phytochemicals, Chem Sci Rev Lett. 2021;10 (39):396-401.
- Girish K., Shankara Bhat S. Phomopsis azadirachtae – The Die-Back of Neem Pathogen. EJBio. 2008b:4(3):112-119.
- Sateesh M. K., Shankara Bhat S., Devaki N. S. Phomopsis azadirachtae nov. from India. Mycotaxon. 1997:65:517-520.
- Shankara Bhat S., Sateesh M. K., Devaki N. S. A New Destructive Disease of Neem (Azadirachta indica) Incited by Phomopsis azadirachtae. Curr Sci. 1998;74:17-19.
- Girish K., Shankara Bhat S. Raveesha K. A. In vitro Screening of Systemic Fungicides against Phomopsis azadirachtae, the Incitant of Die-back Disease of Neem. Arch Phytopathol Pl Protect. 2009:42:256-264.
CrossRef - Fenta L., Mekonnen H. Microbial Biofungicides as a Substitute for Chemical Fungicides in the Control of Phytopathogens: Current Perspectives and Research Directions, Scientifica. 2024;5322696. DOI.10.1155/2024/5322696
CrossRef - Colagiero M., Pentimone I., Rosso L., Ciancio, A. Soil Biodiversity and Microbial Antagonism for Suppression of Plant-Parasitic Nematodes. CABI Reviews. 2024;19(1). DOI.10.1079/cabireviews. 2024.0048
CrossRef - Kalit S. N. D. M., Zain W. Z. W. M., Ghazali, A. H., Ramli N. W., Aziman N., Hamzah F., Hamid N. A., Musa, S. A. N. C. Antifungal Properties of Andrographis paniculata Extracts as Potential Biofungicides: a Review. Food Res. 2024;8(3):494-504. DOI. 10.26656/fr.2017.8(3).246
CrossRef - Garnayak N., Senapati S., Patra R., Panda S., Jena G., Mishra S. In vitro Antibacterial Activity of Andrographis paniculata Aqueous, Methanolic and Ethanolic Leaf Extracts. The Pharma Innov J. 2023:12(4):573-577.
- Aneja K.R. Experiments in Microbiology, Plant Pathology, Tissue Culture and Mushroom Cultivation. 2nd New Delhi: Vishwa prakashan; 1996.
- Lahlali R., Ezrari S., Radouane N., Kenfaoui J., Esmaeel Q., El Hamss H., Belabess Z., Barka E. A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms. 2022;10(3):596. DOI.10.3390/microorganisms10030596
CrossRef - Ayaz M., Li C. H., Ali Q., Zhao W., Chi Y. K., Shafiq M., Ali F., Yu X. Y., Yu Q., Zhao J. T., Yu, J. W., Qi, R. D., Huang, W. K. Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules. 2023;28(18):6735. DOI. 10.3390/molecules28186735
CrossRef - Al-Nadabi H. H., Al-Buraiki N. S., Al-Nabhani A. A., Maharachchikumbura S.., Velazhahan R.., Al‐Sadi A. In vitro Antifungal Activity of Endophytic Bacteria Isolated from Date Palm (Phoenix doctylifera) Against Fungal Pathogens Causing Leaf Spot of Date Palm. Egypt J Biol Pest Control. 2021;31:65. DOI: 10.1186/s41938-021-00413-6
CrossRef - Hussein S.N., Safaie N., Shamsbakhsh M., Al-Juboory H. H. Harnessing Rhizobacteria: Isolation, Identification, and Antifungal Potential Against Soil Pathogens. Heliyon. 2024;10(15): e35430. DOI: 1016/j.heliyon.2024.e35430.
CrossRef - Goswami M., Deka S. Isolation of a Novel Rhizobacteria having Multiple Plant Growth Promoting Traits and Antifungal Activity against Certain Phytopathogens. Microbiol Res. 2020; 240: 126516. DOI: 10.1016/j.micres.2020.126516
CrossRef - Erhonyota, C., Edo, G.I., & Onoharigho, F.O. (2022). Comparison of poison plate and agar well diffusion method determining the antifungal activity of protein fractions. Acta Ecol Sin. 2023;43(4): 684-689. DOI. 10.1016/j.chnaes.2022.08.006
CrossRef - Jabborova D., Mamarasulov B., Davranov K., Enakiev Y., Bisht N., Singh S., Stoyanov S., Garg A. P. Diversity and Plant Growth Properties of Rhizospheric Bacteria Associated with Medicinal Plants. Indian J Microbiol. 2024;64:409–417. DOI. 10.1007/s12088-024-01275-w
CrossRef

