Introduction
Plants are exposed to numerous abiotic stresses such as low temperature, drought, salt, heat, floods, oxidative stress and heavy metal toxicity throughout their life cycle. Amongst all this, salinity is the most typical abiotic stress.1 Salt stress is a significant growth restrictive element for most non-halophyte plants. The plant growth is ultimately abridged by salinity stress even though plant species diverge in their tolerance to salinity.2 Salt stress affects countless facets of plant metabolism and, as a result, growth and yield are suppresed. Surplus salt in the soil solution may unfavorably distress plant growth through whichever osmotic inhibition of water uptake by roots or definite ion effects.
The zone exaggerated by salinity in the world shields about 400 million hectares, of which 54 million are found in South and Southeast Asia.3 In spite of the widespread collected works there is still a debate with respect to the mechanisms of salt tolerance to plants.4 This concern reflects an important fear within the agricultural sector and rural communities, of facing a strong decrease in soil fertility and productivity due high salinity resulting from sea intrusion. In saline soil, crop growth is severely restricted. Elevated levels of soil salinity can impede seed germination and seedling development, due to the collective consequences of high osmotic potential and specific ion toxicity.5 It is thought that the depressive effect of salinity on germination could be related to a decline in endogenous levels of hormones.6
However, incorporation of Trichoderma during seed bio priming treatments in many cereal and vegetable crops has resulted in increased levels of plant growth hormones and improved seed performance.7 Bio priming is a method of biological seed treatment that discusses amalgamation of seed hydration and seed inoculation with beneficial organisms to safeguard seed. The procedure aids seeds to uniformly germinate even under adversarial soil conditions.8 Biocontrol agent, Trichoderma, releases a variety of compounds that induce resistance responses to biotic and abiotic stresses.9,10 Several studies have shown that root colonization by Trichoderma harzianum results in increased level of plant enzymes, including various peroxidase, chitinases, β-1,3-glucanases, lipoxygenase-pathway hydro peroxide lyase and compounds like phytoalexins and phenols to provide durable resistance against stress.11 Therefore, present research work aimed to find out the potential isolates of Trichoderma with high biocontrol potential and plant growth promotion ability by improving the soil quality.
Trichoderma species play an imperative role in salinity reduction; it has antimicrobial potential to colonize diverse substrates under different environmental conditions.12 The seed pretreatment with Trichoderma species upsurges indole-3-acetic acid (IAA) or 1-aminocyclopropane-1-carboxylate (ACC) contents in plants under stress and induces stress tolerance leading to an escalation in plant growth.13 The accretion of reactive oxygen species (ROS) is an illustrious consequence of salt stress. Plants develop scavenging mechanisms that include both enzymatic and non-enzymatic antioxidants to effectively mitigate the ROS damage. The major enzymatic systems for ROS scavenging mechanisms, superoxide dismutase (SOD), peroxidases (POD), and catalase (CAT), are also significant parameters for evaluating salt resistance in plants.14 These ROS scavenging mechanisms, interceded by antioxidant enzymes, are the first line of defense in contrast to salt stress and directly reflect the effects of salt stress on plants.15 To sustain the equilibrium between ROS development and interception and to alleviate the negative effects of salt stress on plant metabolism and growth, an effective antioxidant capability is crucial.
Material and Methods
Isolation of Biocontrol Agent from Rhizosphere Soil
From the rhizosphere of chickpeas (Cicer arietinum), 76 soil samples were collected and 21 isolates of Trichoderma were recovered (Table 1). In order to isolate species, soil samples were dissolved in 9 millilitres of sterilized distilled water and serially diluted in sterile Petri plates having sterile Potato Dextrose Agar (PDA) medium and Trichoderma specific medium (0.2 grams of MgSO4.7H2O, 0.9 grams of K2HPO4, 0.15 grams of KCl, 3.0 grams of NH4NO3, 3.0 grams of glucose, 15 grams of Agar, 0.15 grams of Rosebengal, 0.25 grams of Chloramphenicol, 1000 millilitres of Distilled water, pH-6.5).16 1% streptomycin added to the medium to prevent the growth of bacteria. For growth, the plates were maintained at 28 ± 1 °C. Every isolate of Trichoderma was sub cultured and kept in Potato Dextrose Medium (PDA) as a single spore pure culture. Microscope was used for morphological characterisation.17 In order to identify the species, the taxonomic and morphological keys supplied by Bisset.18,19
Nucleic acid extraction, PCR amplification sequencing and DNA analysis of Trichoderma isolates
PCR and Sanger sequencing was used to amplify ITS region 1and 4 of the rRNA gene cluster, and the translation elongation factor 1-alpha (tef1), TEF 728R (CAT CGA GAA GTT CGA GAA GG) & 986F (TAC TTG AAG GAA CCC TTA CC).
The raw sequence reads of ITS1 and ITS4, tef were checked for quality, trimmed, manually edited and assembled using CLC Genomics Workbench 7.5 (CLCBio, Aarhus, Denmark).
The ITS sequences of the isolated Trichoderma isolates were aligned with the reference sequences of Trichoderma obtained from NCBI database using Clustal W software and phylogenetic relationships were obtained.
Table 1: Trichoderma identified and submitted to NCBI having Accession number as follows:
| S.No | Area/ District | Rhizospheric soil | Isolate | Trichoderma identified | ITS Gene | TEF Gene |
| 1. | Rajoula chitrakoot, MP | Chickpea | Tr1 | Trichoderma asperellum | OP938770 | OP948258 |
| 2. | Rajoula chitrakoot, MP | Chickpea | Tr2 | Trichoderma asperelloides | OP938771 | OP948259 |
| 3. | Badausa, UP | Chickpea | Tr3 | Trichoderma brevicompactum | OP938772 | OP948260 |
| 4. | Fatehpur roshami, UP | Pigeonpea | Tr4 | Trichoderma asperellum | OP938773 | OP948261 |
| 5. | Mangwada, unnao | Chickpea | Tr5 | Trichoderma harzianum | OP938774 | OP948262 |
| 6. | Chitrakoot | Chickpea | Tr6 | Trichoderma longibrachiatum | OP938775 | OP948263 |
| 7. | Footera(Orccha)Jhansi, UP | Pigeonpea | Tr7 | Trichoderma asperellum | OP938776 | OP948264 |
| 8. | Footera(Jhansi) | Chickpea | Tr8 | Trichoderma asperellum | OP938777 | OP948265 |
| 9. | Naramau, Kanpur, UP | Pigeonpea | Tr9 | Trichoderma longibrachiatum | OP938778 | OP948266 |
| 10. | IIPR, Kanpur, UP | Chickpea | Tr10 | Trichoderma longibrachiatum | OP938779 | OP948267 |
| 11. | IIPR, Kanpur, UP | Chickpea | Tr11 | Trichoderma afroharzianum | OP938780 | OP948268 |
| 12. | IIPR, Kanpur, UP | Chickpea | Tr12 | Trichoderma asperellum | OP938781 | OP948269 |
| 13. | Kanpur | Chickpea | Tr13 | Trichoderma asperellum | OP938782 | OP948270 |
| 14. | Kanpur | Chickpea | Tr14 | Trichoderma asperellum | OP938783 | OP948271 |
| 15. | Kanpur | Chickpea | Tr15 | Trichoderma asperellum | OP938784 | OP948272 |
| 16. | Kanpur | Chickpea | Tr16 | Trichoderma asperellum | OP938785 | OP948273 |
| 17. | Kanpur | Chickpea | Tr17 | Trichoderma asperellum | OP938786 | OP948274 |
| 18. | Kanpur | Chickpea | Tr18 | Trichoderma asperellum | OP938787 | OP948275 |
| 19. | Kanpur | Chickpea | Tr19 | Trichoderma asperellum | OP938788 | OP948276 |
| 20. | Kanpur | Chickpea | Tr20 | Trichoderma asperellum | OP938789 | OP948277 |
| 21. | Kanpur | Chickpea | Tr21 | Trichoderma asperellum | OP938790 | OP948278 |
Identification of antagonistic activity of Trichoderma isolate
Using the binary culture method, the Trichoderma isolates were assessed for their antagonistic capability against R. bataticola in an in vitro setting.20 Sterilized Petriplates with a 90 mm diameter, 20 ml of sterilized PDA medium, and antagonists were injected at the periphery opposite each other. The plates were then incubated at 28±1°C. Plates injected with pathogens serve as the control. After the pathogen’s development completely covered the plate, the binary culture Trichoderma spp. inoculation was evaluated. Using the following formula21, the suppression impact of all Trichoderma spp. was assessed in terms of Percentage Inhibition in Radial Growth (PIRG) of R. bataticola. PIRG = R1- R2 x 100% / R1 R1 = R. bataticola‘s radial development in the growth of R. bataticola in the presence of the Trichoderma isolates (treatment). The experiment was done in triplicate.
Selection of Thermotolerant and salinity tolerant Trichoderma
Effect of Temperature
Temperature plays an important role in articulating the activity of any biological system; it has great influence on radial growth and sporulation of Trichoderma. Temperature extensively affected the radial growth and sporulation of Trichoderma spp. The capability of Trichoderma spp. to grow at limiting temperature was evaluated by growing the cultures on PDA plates at different temperatures viz., 30, 35 and 40°C. For measuring the radial growth rate, 5 mm mycelial discs of Trichoderma spp. was inoculated on 90 mm potato dextrose agar plates. The plates were incubated at above mentioned temperatures and the radial growth was measured (mm) everyday up to 7 days of inoculation. Experiment was conducted in triplicates.
Effect of salinity
For salt tolerance examination of Trichoderma isolates mycelial discs were sited on PDA medium supplemented with 5%, 10% and 15% NaCl, respectively and incubated at 28˚C for 7 days. Unit of existence was considered by assessing the colony growth of isolates on media. Three replicates for each concentration was used and PDA without NaCl used as control.22
Evaluation of potential Trichoderma strains on seed germination test and seedling vigour assay
An experiment was carried out to evaluate the ability of twenty one nominated effectual isolates on seed germination and confirmed for their plant growth promotion capability by the standard roll towel method.23 in a growth chamber. Trichoderma were grown in Potato Dextrose broth medium for 7 days. Spore suspension was made by calculating optical density. Seeds of chickpea were surface sterilized for 5 min with 0.1% mercuric chloride, bathed with sterilized distilled water (SDW) and saturated in Trichoderma spore suspension (2×10 8 cfu ml-1) using Tween 80 for 24h and sterilized Potato Dextrose broth served as control. Then blot dried chickpea seeds placed in wet blotters and incubated in growth chamber maintained at 28±20C and 95±3% Relative humidity. Ten replicates of each strain were designed. The percentage of germination was recorded at seventh day. Length of root and shoot of the seedling were measured separately at 7 days.24 Plant growth promotion of chickpea seedling was evaluated using Vigour Index (VI). VI = per cent germination x mean total length of seedling (root length + shoot length).
Pot evaluation of Trichoderma isolates
The biocontrol potential of 21 Trichoderma isolates was further evaluated under field conditions during the growing season of 2021-2022. For pot evaluation, the trial was conducted in a completely randomized design with 22 treatments and three replications. All the Trichoderma isolates were multiplied on farm yard manure separately and used as inoculums in the field. Dose of Trichoderma application was 1kg FYM (6X 1 05 C .F. U) g-J was applied at the base of each plant. After three months various growth parameters like germination percentage, root length, shoot length, vigour index, no. of secondary roots, no. of internodes, chlorophyll, flavonoids etc was measured.
Statistical Analysis
The experiments were performed with three replicates. The analysis of variance (ANOVA) was performed using OPSTAT software. Mean values among treatments were compared by the least significant difference using critical difference at 95% level of confidence (p<0.05%). For graphical representations and descriptive statistical analysis, Microsoft Excel was used.
Results and Discussion
Evaluation of antagonistic effect of Trichoderma isolates against Rhizoctonia bataticola using dual culture tests showed that 11 isolates Tr5, Tr6, Tr8, Tr9, Tr10, Tr11, Tr13, Tr16, Tr17, Tr20 and Tr21 reduced the mycelial growth of R. bataticola more than 70% (Fig1). Maximum mycelial inhibition was 76.48% by isolate Tr17 and 75.85 % by Tr13. Ten isolates inhibited mycelial growth of R. bataticola more than 50% but less than 70%. The isolates overgrew on the R. bataticola colonies which had irregular morphology and were lysing indicating the incidence of strong mycoparasitism.25
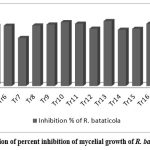 |
Figure 1: Graphical presentation of percent inhibition of mycelial growth of R. bataticola by Trichoderma isolates |
Salt and temperature tolerant isolates of Trichoderma spp.
The inoculation of isolates on the PDA medium supplemented with 5, 10 and 15% NaCl. Seven days after incubation at 28OC, there was growth in all the isolates in medium supplemented with 5% NaCl whereas there was retardation in growth in all the isolated in the medium supplemented with 15% NaCl. The growth in medium supplemented with 10% NaCl gave indication of salt tolerance. Less than 60% reduction in growth was observed in four isolates viz., Tr11, Tr10, Tr20 and Tr13. whereas reduction in growth between 60-80% was observed in 5 isolated, between 80-90% in 7 isolates, between 90-95% in 5 isolates. Highly sensitive to salt were five isolates. (Table 2)
Table 2: Salt Tolerance by Trichoderma isolates
| Salt Tolerance perc reduction | |
| Tr11 | ++++ |
| Tr10 | ++++ |
| Tr20 | ++++ |
| Tr13 | ++++ |
| Tr17 | +++ |
| Tr15 | +++ |
| Tr14 | +++ |
| Tr16 | +++ |
| Tr12 | +++ |
| Tr4 | ++ |
| Tr6 | ++ |
| Tr2 | ++ |
| Tr1 | ++ |
| Tr7 | ++ |
| Tr21 | ++ |
| Tr3 | ++ |
| Tr9 | + |
| Tr18 | + |
| Tr5 | + |
| Tr19 | + |
| Tr5 | + |
Temperature plays important role in growth and sporulation of Trichoderma spp. The growth was measured at three temperatures 30, 35 and 40⸰C. All the isolates grew well at 30⸰C, whereas the all the isolates failed to grow at 40⸰C. Therefore, the growth of isolates was taken into consideration for distinguishing thermotolerant isolates. Eight isolates namely Tr20, Tr19, Tr17, Tr10, Tr11, Tr12, Tr13 and Tr9 were most thermotolerant with mycelium growth of more than 4cm at 35⸰C. Seven isolates were having growth between 3-3.9 cm, seven isolates were having growth between 2-2.9cm, four isolates were having growth between 1-9 cm and only two isolates Tr7 and Tr8 were most sensitive to temperature having growth less than 1 cm at 35⸰C. (Table 3)
Table 3: Thermo tolerance by Trichoderma isolates
| Thermotolerant | |
| Tr20 | ++++ |
| Tr19 | ++++ |
| Tr17 | ++++ |
| Tr10 | ++++ |
| Tr11 | ++++ |
| Tr12 | ++++ |
| Tr7 | ++++ |
| Tr9 | ++++ |
| Tr5 | +++ |
| Tr14 | +++ |
| Tr8 | +++ |
| Tr21 | +++ |
| Tr1 | +++ |
| Tr16 | +++ |
| Tr2 | +++ |
| Tr6 | ++ |
| Tr4 | ++ |
| Tr18 | ++ |
| Tr15 | ++ |
| Tr3 | + |
| Tr13 | + |
Scale zone measured in mm
Salt Tolerance (Below 60= +++), (60-80= ++), (80-90= +), ( 90<= -)
Thermo tolerance (>40= ++++), (30-39= +++), (20-29= ++), (10-19= +), (<10= -)
Seed Biopriming
Bio priming is a method of biological seed treatment that discusses amalgamation of seed hydration and seed inoculation with beneficial organisms to safeguard seed.8 Biocontrol agent, Trichoderma, proclaims a diversity of compounds that persuade resistance responses to biotic and abiotic stresses.9,10
Evaluation of potential Trichoderma strains on seed germination test and seedling vigour assay
The experiment was conducted to assess the influence of 21 selected efficient isolates on seed germination and tested for their plant growth promotion capacity by the standard roll towel method.23 in growth chamber. Trichoderma were grown in Potato Dextrose broth medium for 7 days. Spore suspension was made by calculating optical density. Chickpea seeds were surface sterilized for 5 min with 0.1% mercuric chloride, rinsed with sterilized distilled water (SDW) and soaked in Trichoderma spore suspension (2×10 8 cfu ml-1) using Tween 80 for 24h and sterile Potato Dextrose broth served as control. Then the seeds were blot dried, placed in wet blotter paper and incubated in growth chamber at 28±20C and 95±3% Relative humidity. Each treatment was replicated 10 times. The percentage of germination was recorded at seventh day. Seedlings were taken length of root and shoot measured separately at 7 days.24 Plant growth promotion of chickpea seedling was measured using Vigour Index (VI). VI = per cent germination x mean total length of seedling (root length + shoot length).
Table 4: Screening of potential plant growth promoting Trichoderma spp. for seedling growth in chickpea
| Isolates | Shoot Length(cm) | Germination Percentage | Root Length (cm) | No. of Secondary Roots | Vigour Index |
| Tr17 | 9.12 | 100 | 17.68 | 22 | 2680 |
| Tr10 | 6.88 | 100 | 15.85 | 10 | 2273 |
| Tr20 | 6.76 | 100 | 14.85 | 16 | 2161 |
| Tr11 | 6.9 | 90 | 16.3 | 15 | 2088 |
| Tr8 | 5.3 | 100 | 14.12 | 16 | 1942 |
| Tr13 | 7.55 | 90 | 13.59 | 19 | 1902.6 |
| Tr19 | 4.68 | 90 | 14.37 | 12 | 1714.5 |
| Tr4 | 6.1 | 90 | 11.1 | 13 | 1548 |
| Tr16 | 4.95 | 90 | 11.68 | 11 | 1496.7 |
| Tr2 | 5.52 | 90 | 11.1 | 11 | 1495.8 |
| Tr18 | 4.21 | 90 | 12.1 | 14 | 1467.9 |
| Tr9 | 4.17 | 90 | 11.18 | 12 | 1381.5 |
| Tr12 | 6.31 | 90 | 8.97 | 13 | 1375.2 |
| Tr7 | 5.23 | 90 | 9.5 | 10 | 1325.7 |
| Tr6 | 4.25 | 80 | 12.1 | 12 | 1308 |
| Tr21 | 5.54 | 80 | 10.78 | 11 | 1305.6 |
| Tr3 | 5.11 | 100 | 7.9 | 11 | 1301 |
| Tr5 | 5.25 | 80 | 10.67 | 8 | 1273.6 |
| Tr14 | 3.21 | 90 | 10.7 | 10 | 1251.9 |
| Tr1 | 4.12 | 90 | 9.14 | 9 | 1193.4 |
| Tr15 | 3.56 | 80 | 8.8 | 9 | 988.8 |
| Control | 5.65 | 90 | 8.75 | 11 | 1296 |
A lone treatment of seed or plants that might instantaneously converse resistance to biotic stresses (disease) and abiotic stresses would be of prominence to agricultural plant production. This report validates that seed treatments with Trichoderma spp. are proficient of assuaging abiotic and physiological stresses in seed and seedlings. A total of twenty one isolates were evaluated for growth promotion using paper towel method on chickpea wherein 08 strains were found effective for growth promotion of chickpea seedling. Tr17, Tr13, Tr10 and Tr20 were found most effective for growth promotion of chickpea.
Evaluation of Trichoderma isolates as plant growth promoters under pot culture.
All the isolates of Trichoderma isolates were multiplied on farm yard manure separately for the plant growth under pot culture conditions. Dose of Trichoderma application was 1kg FYM (6X 1 05 C .F. U) g-J was applied at the base of each plant. After three months various growth parameters like germination percentage, root length, shoot length, vigour index, no. of secondary roots, chlorophyll, flavonoids etc were measured. The plant vigour was improved in all Trichoderma treated plants compared to control. However, more plant vigour was observed in plants treated with Trichoderma isolates Tr17, Tr13, Tr20, and Tr11. These isolates also improved significantly the secondary roots, fresh weight and vigour of the plants. Other parameter like Nitrogen Biological Index, chlorophyll content, flavonoids and SPAD were recorded. These parameters improved in Trichoderma treated plants compared to control plants. Based on these four parameters, above four isolates were also found most effective.
Table 5: Evaluation of Trichoderma as plant growth promoter under pot culture
| Trichoderma Isolates |
Roots (cm) |
Shoots (cm) |
Secondary Root |
Germination | Vigour |
Fresh weigh (Mean) cm |
Nitrogen Balance Index |
CHL | FLAV | Soil Plant Analysis Development |
| Tr17 | 12.00 | 32.67 | 22 | 60.0 | 38 | 40.22 | 46.10 | 28.00 | 22.12 | 34.50 |
| Tr13 | 12.30 | 33.17 | 23 | 61.5 | 39 | 41.12 | 62.20 | 25.10 | 16.10 | 35.30 |
| Tr20 | 14.60 | 28.07 | 21 | 73.0 | 41 | 45.04 | 34.00 | 32.70 | 34.00 | 59.10 |
| Tr11 | 14.73 | 27.87 | 21 | 73.7 | 41 | 45.30 | 16.10 | 14.10 | 0.84 | 48.20 |
| Tr10 | 15.10 | 26.13 | 21 | 75.5 | 41 | 45.62 | 31.40 | 33.60 | 4.20 | 25.20 |
| Tr8 | 14.77 | 31.90 | 23 | 73.8 | 43 | 46.73 | 43.70 | 37.80 | 43.70 | 42.60 |
| Tr19 | 17.50 | 28.37 | 23 | 87.5 | 46 | 52.23 | 17.90 | 13.20 | 1.30 | 45.10 |
| Tr4 | 9.02 | 31.53 | 20 | 45.1 | 32 | 32.55 | 28.30 | 25.30 | 28.30 | 10.20 |
| Tr16 | 10.20 | 27.80 | 19 | 51.0 | 33 | 34.20 | 8.00 | 12.30 | 0.79 | 6.40 |
| Tr12 | 9.73 | 27.93 | 19 | 48.7 | 32 | 33.10 | 45.45 | 23.20 | 54.30 | 26.00 |
| Tr2 | 14.47 | 28.90 | 22 | 72.3 | 41 | 45.00 | 10.60 | 8.30 | 10.60 | 16.90 |
| Tr3 | 9.40 | 24.90 | 17 | 47.0 | 30 | 31.28 | 52.60 | 21.40 | 0.41 | 51.10 |
| Tr7 | 8.37 | 33.80 | 21 | 41.8 | 32 | 31.72 | 29.30 | 28.60 | 0.98 | 31.50 |
| Tr5 | 15.47 | 22.30 | 19 | 77.3 | 40 | 45.24 | 43.10 | 18.20 | 43.10 | 56.80 |
| Tr21 | 12.97 | 23.23 | 18 | 64.8 | 35 | 39.44 | 71.40 | 28.50 | 0.40 | 46.70 |
| Tr6 | 10.00 | 27.33 | 19 | 50.0 | 32 | 33.56 | 44.10 | 24.70 | 0.56 | 49.30 |
| Tr14 | 11.63 | 23.83 | 18 | 58.2 | 33 | 36.38 | 28.80 | 30.70 | 1.07 | 26.30 |
| Tr15 | 15.03 | 21.63 | 18 | 75.2 | 38 | 43.96 | 30.00 | 27.80 | 30.00 | 48.90 |
| Tr1 | 11.47 | 25.20 | 18 | 57.3 | 34 | 36.43 | 23.70 | 22.80 | 0.96 | 45.80 |
| Tr9 | 13.53 | 19.87 | 17 | 67.7 | 35 | 39.70 | 35.80 | 28.50 | 0.80 | 63.30 |
| Tr18 | 12.00 | 29.20 | 21 | 60.0 | 37 | 39.07 | 25.50 | 12.80 | 0.52 | 16.80 |
| Control | 9.00 | 18.33 | 14 | 45.0 | 26 | 28.11 | 30.40 | 14.60 | 2.50 | 19.30 |
Most Ascomycetes fungi which naturally exist have growth maximum at 30°C.26 to 35°C.27 Nevertheless in contrast to those, the present exploration has acknowledged particular thermotolerant Trichoderma isolates with highest growth rate at 40°C. In many Trichoderma isolates growth was slow above 35°C and ceased at 40°C. In addition, many morphological variations were apparent at various temperatures. At 35°C, their colonies were abnormal with irregular margin and inadequate sporulation, while at 40°C they failed to sporulate even after 7 days of incubation. Some isolates such as Tr20, Tr19, Tr17, Tr10, Tr11, Tr12, Tr7 and Tr9 sporulated at and above 40⸰C (Table 2).
The Trichoderma has an important role in metabolic course of action of host plants that could pass on salinity tolerance.28 NaCl affects the plant growth negatively.29,30 However, Trichoderma spp. found to mitigate the damaging effects of NaCl stress in chickpea. In the present investigation Trichoderma spp. showed a high range of NaCl tolerance. The highest tolerance was shown by Tr11, Tr10, Tr2 and Tr13. It was observed that at 5% concentration of NaCl, growth and sporulation was good which corroborates with the findings of.31 in tomato. Growth was supported well but sporulation was poor at 10% concentration. At 15% concentration, the growth and sporulation was poor in all the isolates (Table 2). Use of the salt tolerant isolates of Trichoderma spp. could bring salt affected areas under cultivation with sustainable crop production.
In vitro testing of 21 isolates as bio this growth promotion has also been supported by improved germination and seedling vigour of chickpea with in vitro testing of 21 isolates of Trichoderma. Pot evaluation also indicated that growth parameters and the plant vigour was generally more in pots treated with Trichoderma isolate.
The results of the current study exhibited that genetic diversity exists among isolates of Trichoderma under PCR-based genetic markers including ITS (Internal Transcribed Spacer) & TEF (Translation Elongation Factor). Several researchers have used to assess genetic diversity in Trichoderma spp.32 The genetic variability within 21 isolates of Trichoderma collected from different geographic locations and culture collections and their Phylogenetic analysis were done with the help of the sequence data obtained. Five species of Trichoderma isolates were identified by molecular methods which were further characterized into three main clades by sequence analysis.
Numerous researchers have described that the decomposition of plant’s litter reinforced higher colonization of microbes because of richness in nutrients.33 Several Trichoderma isolate were found to be the promising among all the tested parameters (Table 3). A large number of field trials are required to understand the biocontrol potential of Trichoderma as biocontrol agent for the control of disease and plant growth. Therefore, location specific Trichoderma isolates would be beneficial to chickpea productivity both in terms of disease protection and increased productivity of chickpea. From this study, the Trichoderma isolates having multiple characters for biotic and abiotic resistance with plant growth promotion could be used for healthy crop production across the location where dry root rot pathogen causing yield loss in chickpea.
Conclusion
Overall, the findings of this study suggest that the selected Trichoderma isolates show promise as plant growth promoting and biocontrol agents against Rhizoctonia solani causing Dry root rot of chickpea
Our results provide a basis for future incorporation of biological control agents into management strategies to control.
Acknowledgment
Authors are thankful to the Director, ICAR-Indian Institute of Pulses Research (IIPR), Kanpur-208024, India and Vice Chancellor and Dean Agriculture, Bhagwant University, Ajmer, Rajasthan, India for providing the necessary facilities and support during the experimentation.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability statement
The datasets generated and/or analysed in the current study entitled “Impact of Trichoderma strains isolated from pulses rhizosphere on plant growth promotion of chickpea” are available from the corresponding author upon reasonable request.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Consent Statement
The article entitled “Impact of Trichoderma strains isolated from pulses rhizosphere on plant growth promotion of chickpea “does not contain any human or animal rights
Consent for Publication
All the authors have provided their consent for publication in this Journal. Attached
Author Contribution
Utkarsh Singh Rathore: All research work during Ph.D
Rudra Pratap Singh: Ph.D advisor, worked under him during Ph.D, helped in designing my research work
Sonika Pandey: helped in data collection & related work and paper writing.
R.K. Mishra: worked under him during my research work
Refernces
- Mahajan S, Tuteja NK. “Cold, salinity and drought stresses: an overview.” Archives of biochemistry and biophysics. 2005; 444(2): 139-58.
CrossRef - Munns R, Annie T. “Whole-plant responses to salinity.” Australian Journal of Plant Physiology.1986; 13: 143-160.
CrossRef - Akbar MU, Ponnamperuma FN. “Saline soils of South and Southeast Asia as potential rice lands.1982.
- Neumann PM. Inhibition of root growth by salinity stress: toxicity or an adaptive biophysical response? In: Baluska F, Ciamporova M, Gasparikova O, Barlow PW (eds) Structure and function of roots. Kluwer, Dordrecht. 1995; 299–304
CrossRef - Grieve CM, Suarez DL. Purslane (Portulaca oleracea L.): a halophyticcrop for drainage water reuse systems. Plant and Soil. 1997;192: 277-283
CrossRef - Afzal I, Basra SMA, Farooq M, Nawaz A. Alleviation of salinity stress in spring wheat by hormonal priming with ABA, salicylic acid and ascorbic acid. Int J Agr Biol. 2006;8:23–28
- Howell CR. Mechanisms Employed by Trichoderma Species in the Biological Control of Plant Diseases: The History and Evolution of Current Concepts. Plant Dis. 2003;87 (1):4-10. doi: 10.1094/PDIS.2003.87.1.4. PMID: 30812698.
CrossRef - Singh US, Zaidi NW, Joshi D, Varshney S, Khan T. Current status of Trichoderma as bio-control agent. In: Ramanujam B, Rabindra RJ (eds) Current status of biological control ofplant diseases using antagonistic organisms in India. Project Directorate of Biological Control,Bangalore. 2003;13–48
- Cardona R, Rodriguez H. Effects of Trichoderma harzianum fungus on the incidence of the charcoal rot disease on sesame. Rev Fac Agron (LUZ). 2006;23:42–47
- Harman GE, Howell CR, Vitarbo A, Chet I, Lorito M. Trichoderma species –opportunistic, avirulent plant symbionts. Nature Rev Microbiol. 2004;2:43–56
CrossRef - Harman GE. Overview of mechanisms and uses of Trichoderma spp. Phytopathology. 2006; 96;190–194
CrossRef - Cheng L, Xu Z, Zhou X. Application of Trichoderma species increases plant salinity resistance: a bibliometric analysis and a meta-analysis. J Soils Sediments.2023;23:2641–2653. https://doi.org/10.1007/s11368-023-03557-0
CrossRef - Solomon B, Zhang S, Xu B, Li T, Inayat R, Calderón-Urrea, Alejandro.The Role of Trichoderma Species in Plants Response to Salt Stress. Asian Journal of Research in Crop Science. 2021. 10.9734/AJRCS/2021/v6i230114.
- Jomova K, Alomar SY, Alwasel SH. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nano materials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. 2024; 98:1323–1367. https://doi.org/10.1007/s00204-024-03696-4
CrossRef - Mohammad R, Boorboori, Haiyang Z. The Mechanisms of Trichoderma Species to Reduce Drought and Salinity Stress in Plants. Phyton-International Journal of Experimental Botany.2023;92(8): 2261-2281, https://doi.org/10.32604/phyton.2023.029486.
CrossRef - Elad Y. Trichoderma harzianum: A Biocontrol Agent Effective Against Sclerotium rolfsii and Rhizoctonia solani. Phytopathology.1980; 70. 10.1094/Phyto-70-119.
CrossRef - Gams W. Hoekstra E.S. Aptroot A. CBS Course on Mycology. Centraal bureau voor Schimmel cultures, AG Baarn, the Netherlands.1998
- Bissett J. A revision of the genus Trichoderma. II. Infrageneric classification. Can J Bot. 1991;69:2357–2372. doi: 10.1139/b91-297.
CrossRef - Bissett J. A revision of the genus Trichoderma. III. Section Pachybasium. Can J Bot. 1991;69:2373–2417. doi: 1.1139/b91-298.
CrossRef - Ramanathan GM, Sundar S, Vinodhkumar T. Evaluation of antifungal activity of metabolites from Trichoderma species against fungal phytopathogens. Inter J Sci Inn Disc.2013; 3(5): 528-538.
- Gaigole AH, Wagh GN, Khadse AC. Antifungal activity of Trichoderma species against soil borne pathogen. Asiat J Biotechnol Res.2011; 4: 461-465.
- Poosapati S, Ravulapalli PD, Tippirishetty N, Vishwanathaswamy DK, Chunduri S.Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. Spring Pl.2014; 3: 641 doi: 10.1186/2193-1801-3-641
CrossRef - Handbook of tetrazolium testing. Zurich, International Seed Testing Association.1985;72
- Abdul Baki A A, Anderson JD.Vigor determination in soybean by multiple criteria. Cr Sci.1973;13: 630-633.
CrossRef - Rathore US, Singh RP, Pandey S, Mishra RK. Molecular Identification, Antagonistic Assay and Enzyme Profiling of Selected Trichoderma Isolates. Curr Agri Res.2024; 12(2).
CrossRef - Begoude BAD, Lahlali R, Friel D, Tondje, PR, Jijakli, MH. Response surface methodology study of the combined effects of temperature, pH, and a won the growth rate of Trichoderma asperellum. J. Appl. Microbiol.2007; 103, 845-854.
CrossRef - Moustafa A, Abdel-Azeem Ahmed. Some new records to the Egyptian Ascomycetes with a provisional key to their identification. Assiut University Journal of Botany.2006;35. 87-103.
- Kumar K, Manigundan K, Amaresan N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J Basic Microbiol. 2017;57(2):141-150. doi: 10.1002/jobm.201600369. Epub 2016 Nov 8. PMID: 27862082.
CrossRef - Azooz MM, Youssef A M, Ahmad P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. J. Plant Physiol. Biochem.2011; 3:253–264.
CrossRef - Rasool S, Hameed A, Mohamed A, Rehman MU, Siddiqi O, Ahmad, P. Salt stress: Causes, Types and Responses of Plants. In: Ecophysiology and Responses of Plants under Salt Stress.2013.
CrossRef - Mastouri F, Björkman T, Harman G. Trichoderma harzianum Enhances Antioxidant Defense of Tomato Seedlings and Resistance to Water Deficit. Molecular plant-microbe interactions: MPMI.2012; 25. 1264-71. 10.1094/MPMI-09-11-0240.
CrossRef - Rai, M. Isolation of antibiotic producing Actinomycetes from soil of Kathmandu valley and assessment of their antimicrobial activities.2016.
- Bahram M, Põlme S, Kõljalg U, Zarre S, Tedersoo L.Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. NewPhytol.2012;193: 465–473
CrossRef

