Introduction
Weeds are the noxious pests of agricultural land, where they compete with the crop for space, nutrients, sunlight and other growth regulators. Avena fatua., Phalaris minor and Chenopodium album are major weeds found in Madhya Pradesh, India. The potential yield loss of 16.5-43.0% was recorded due to weed infestation which could be 100% if left uncontrolled in wheat field1 in India. Wheat is grown on about 314.5 lakh hectares of land. Growth and yield of wheat crop is essential to feed the present population. Chemical herbicides have been vigorously utilized to reduce the impact of weeds on crop production. The risk and effect of herbicides and their residues on all tropic levels of food chain and environment has increased public concerns about pesticide-free food.
Hence, there is an urgent need to find out an alternative eco-friendly method such as bioherbicide for controlling weed species. Reducing herbicide toxicity and resistance is sought by use of safer bioherbicide microbes on their products2. Biological control for weed management is recognized as a cost-effective, efficient, and environmentally sustainable approach to weed control. Bioherbicidal products obtained from microbes are easily degradable and they cannot persist for long time in the soil3. Several phytopathogenic fungi, including Fusarium oxysporum and Puccinia komarovii var. Glanduliferae, have demonstrated their effectiveness in controlling weeds4,5. On the other hand, Phoma herbarum is a widespread saprobe and pathogen that can be toxic to plants6. Phoma macrostoma, Phoma chenopodia, and Phoma herbarum have all been mentioned as promising biological control agents for various weeds in earlier investigation7. Phoma herbarum has been found to be effective against Parthenium hysterophorus, Lantana camara L., Hyptis suaveolens, and Sida acuta Burm. f.8. Phoma chenopodia was also reported to be effective against Cirsium arvense, Chenopodium album, Mercurialis annua and Setaria viridis9. Phoma macrostoma was reported as a biocontrol agent for dicot weed plants10.
This study sought to determine bioherbicidal capacity of Phoma herbarum to manage potential weeds of wheat field and this is the first account of Phoma herbarum encouraging wheat growth. Further research is required on the formulation of Phoma herbarum to ensure consistent weed control.
Materials and Methods
Sample Collection and Isolation of Phytopathogenic Microorganisms
Affected parts of weeds were collected during winter season in zip lock bags from irrigated wheat fields and weed-infested areas of Indore, India. Infected samples were preserved at 4-8oC, until further isolation of phytopathogenic microorganisms.
Infected samples of the weed were used for the isolation of phytopathogenic microorganisms. Each infected sample was surface sterilized with 1% NaOCl for 1min and cut into 2-4 mm2 pieces. A size of three to four pieces of infected material were placed on potato dextrose agar (PDA) for fungal isolation and nutrient agar (NA) plate for bacterial isolation and the plates were incubated at 28oC for 7 days. After this, pure culture of each isolate was prepared and stored at 4oC till further use.
Detached Leaf Assay for Screening of Isolates for their Bioherbicidal Activity
Detached leaf assay11 was used for the screening of isolates to test their bioherbicidal activity against the weeds.
6mm fungal disc from PDA plate was inoculated in 100-ml Erlenmeyer flasks containing 25ml potato dextrose broth and incubated for 7 days at 27oC, 120 rpm. Inoculum of each bacterial isolate was prepared by inoculating a loopful of pure culture in 100-ml Erlenmeyer flasks containing 25ml nutrient broth. The bacterial cultures were incubated at 30oC for 2 days at 120 rpm.
Freshly collected healthy leaves of weeds (Avena fatua, Chenopodium album, and Phalaris minor) were used for detached leaf assay. Collected leaves were then washed in running tap water, surface sterilized with 1% NaOCl for 45 sec and subsequently washed 3-4 times with autoclaved distilled water. Three leaves of each variety were placed on moist filter paper in different petri plates. Each leaf was treated with 20µl of bacterial or fungal inoculum. Treated leaves were incubated at 22±2oC, under alternating periods of light and dark for 7 days until the formation of necrotic symptoms. Uncultured sterile growth medium was taken as control. All experiments were repeated and data was measured in triplicates. A similar experiment was performed using the selected isolates on other weeds and wheat varieties, Poshan and Tejas.
Biochemical Characterization of Potential Isolates
A qualitative testing approach like a visible hydrolytic zone and change in media colour was used for estimating the biochemical production of hydrolytic enzymes and secondary metabolites by potential isolates. The zone of hydrolysis produced by enzymes such as amylase on starch agar, protease on skimmed milk agar, cellulase on PDA supplemented with 1% CMC, lipase on tributyrin agar were performed using standard process, laccase on PDA supplemented with 0.01% guaiacol12, chitinase on 1% colloidal chitin agar 13, phosphatase on Pikovskaya agar14, xylanase on birch wood xylan and pectinase on pectin agar15. was considered for qualitative assessment. The qualitative production of secondary metabolites such as HCN by Lorck method16, siderophore on CAS agar17, and indole acetic acid by Salkowski method18 was assessed.
Molecular Identification of Potential Isolates
Most promising fungal isolates were identified using molecular identification facilities at the National Fungal Culture Collection of India (NFCCI), Pune, India. The Genomic DNA of each fungus was isolated in pure form. ITS4 and ITS5 primers were used for successful amplification of the ITS-rDNA partial gene19. The sequencing PCR was set up with ABI-bigdye® Terminatorv3.1 Cycle Sequencing Kit. The raw sequence obtained from ABI 3100 automated DNA sequencer was compared with 18S rDNA sequences using BLAST program.
Effect of Phoma herbarum R21 in Pre-Germination and Post-Gemination Test of Weeds and Wheat.
Culture Preparation
Fungal isolate selected after detached leaf assay is identified as Phoma herbarum R21 and used in further experiments. Phoma herbarum was inoculated on PDA and incubated at 22±2oC for 8 days. The spores were collected by flushing the plate with 10mL of sterile 0.5% Tween-20 solution, followed by filtration through muslin cloth. Spores were calculated using hemocytometer and suspension concentration of 107 spores/mL was maintained using sterile distilled water.
Pre-Germination Treatment Using Fungi
Seeds of three weed varieties such as Avena fatua, Phalaris minor, and Chenopodium album were obtained from the Directorate of Weed Research, Jabalpur, India. Two varieties of Durum wheat Poshan HI8663 and Tejas HI8768 were collected from Kasturbagram Rural Institute Indore, India.
All seeds were washed under running tap water, surface sterilized with 1% NaOCl for 45 seconds followed by rinsing 3-4 times with autoclaved distilled water. A total of 20 seeds of each variety were placed on a moist filter paper layer over cotton in each autoclaved petri plate. The seeds of weed and wheat were treated with 1 ml of fungal spore suspension (107 spores/ml in 0.05% Tween 20). Seeds treated with 0.05% Tween 20, were used as control. The treated seeds were grown for 7 days at 20 ± 2oC under 12hrs alternating conditions of light and dark. Growth parameters such as germination (%), shoot and root length (cm), and fresh biomass of plant (mg) were recorded. The experiment was performed in triplicate. The reduction in weed growth was recorded in the form of growth inhibition percentage (GIP) as given in the formula.

Seedling growth of wheat varieties were examined by calculating growth percentage (GP) as compared to control using given formula. Control was taken as 100% growth.

Post-Germination Treatment Using Fungi
Detached leaf assay as described in this paper was used for post-germination testing against the weeds and wheat leaves. Treated and control sets were incubated for 7 days and phytotoxicity was recorded as positive (+, symptomatic), negative (-, asymptomatic).
Effect of Phoma herbarum R21 CFCF in Pre-Germination and Post-Gemination Test of Weeds and Wheat.
Cell Free Culture Filtrate Preparation
1000 ml Erlenmeyer flasks containing 500 ml PDB were seeded with 6mm fungal discs from 5-day old culture plate of Phoma herbarum. The cultured medium was collected on 8th, 11th and 14th day, under aseptic conditions. The cultured medium was centrifuged at 8000 rpm and pre-filtered through a pre-weighed Whatman filter paper no. 1 and finally filtered using a 0.45µm syringe filter 20 (Himedia). This is to ensure a spore and filament-free CFCF without blocking the syringe filter.
Pre-Germination Treatment Using CFCF
Sterilised seeds of different weeds and wheat varieties were treated with 2ml of CFCF. CFCF (8th, 11th and 14th day) were added to different petri plates containing sterilized seeds and incubated under light (12 h) followed by dark (12 h) at 20°C for 7 days. Germination (%), shoot length (cm), root length (cm), fresh weight (mg) and dry weight (mg) of seedlings were examined in triplicate. Vigour index (plant height in cm x germination %) of seeds was calculated to evaluate activity and performance of seeds. Control treatments were carried out using sterile PDB medium21. The reduction in growth was recorded in the form of growth inhibition percentage.
Post-Germination Treatment Using CFCF
Detached leaf assay was used to test the effect of CFCF on both weeds and wheat leaves. For this 10μL of 8th, 11th and 14th day CFCF was applied on leaves of targeted weed and wheat varieties. Treated and control sets were incubated for 7 days and phytotoxicity was recorded as positive (+, symptomatic) and negative (-, asymptomatic).
Antifungal Activity of Phoma herbarum R21
The selected isolate of Phoma herbarum R21 was tested against 5 fungal phytopathogen viz. Rhizoctonia solani, Sclerotium rolfsii, Bipolaris oryzae, Curvularia lunata and Fusarium oxysporum by dual culture plate technique22 with some slight modifications. Antifungal activity was recorded after incubation of 4 days as percentage inhibition of radial growth (PIRG) and was calculated using the formula as given below23

Statistical Analysis
The triplicate values of each experiment were analysed using MS Excel version 2304 and analysed data are presented as mean ± standard deviation.
Results
Sample Collection and Isolation of Phytopathogenic Microorganisms
A total of 19 samples were collected from four different locations, including two farmlands and two areas surrounding university and college campuses. Morphologically different twelve bacteria and thirty-one fungi were isolated from the diseased weed tissues (Avena fatua, Parthenium, Mauritius grass, Oxalis, Chenopodium album, Amaranthus sp., Euphobia, Lantana, Taraxacum officinale and Apluda sp.) and soil samples, collected from different locations in Indore (Table 1).
Table 1: Sample collection sites and collected weeds.
|
Location |
Coordinates |
Infected Weeds / Soil |
|
|
Latitude |
Longitude |
||
|
Wheat Field, KVK Kasturbagram, Indore |
22.643031 |
75.8893632 |
Avena Fatua (wild oats) Apluda sp. (Mauritian grass) Chenopodium album (Bathua) Parthenium hysterophorus (Gajar ghas) Soil (organic land) |
|
IARI, Regional Centre Indore. |
22.719568 |
75.857727 |
Euphorbia sp. (Doodhi) Amaranthus sp, (Green amaranth) Taraxacum officinale (Dandelion) Soil (low weed density area) |
|
Devi Ahilya University, Indore (DAU) |
22.687061 |
75.8724644 |
Lantana Camara (Panchfooli) Fumaria parviflora (Shahatra) Cynodon dactylon (Doob) |
|
Maharaja Ranjit Singh College, Indore |
22.678901 |
75.8803770 |
Euphorbia sp. (Doodhi) Oxalis (Teen pattia) |
Detached Leaf Assay for Screening of Isolates for their Bioherbicidal Activity
The detached leaf assay of isolates shows the initial symptoms of the disease in the form of necrosis and growth of the causal organism. The development of brownish irregular spots with a chlorotic halo around them, after 48 hours post-treatment (HPT) in three test sets. Four out of the twelve bacterial isolates (DGL1, DGL2, DGL3 and DGL 5A) tested on various weeds were found to be phytotoxic (Table 2). Moreover, among the fungal isolates, two fungal isolates DGL7A and 8C were remarkably infected all three targeted weeds within seven days of treatment compared to control. However, on all the weed leaves DGL 8C developed early symptoms in 36 hours as compared to 48 hours for DGL 7A.
Table 2: List of potential isolated screened by detached leaf assay
|
S.No. |
Isolates |
Avena fatua |
Phalaris minor |
Chenopodium album |
|
1 |
DGL 1 |
+ |
– |
– |
|
2 |
DGL2 |
+ |
– |
– |
|
3 |
DGL3 |
– |
– |
+ |
|
4 |
DGL5A |
– |
– |
+ |
|
5 |
DGL7A |
+ |
+ |
+ |
|
6 |
DGL8C |
+ |
+ |
+ |
(+) indicates infection, (-) indicates no infection
Molecular Identification and Biochemical Characterization of Potential Isolates
The selected fungal isolates, DGL 7A and DGL 8C from leaves of Mauritius grass showed 100% sequence similarity with Phoma herbarum KNESO2 and R2 respectively, based on the analysis of ITS region of rDNA sequences. Identified isolates come under fungal plant pathogen division of Ascomycota. The rDNA sequence of Phoma herbarum R21 was submitted to NCBI GenBank with accession ID ON705696.
Biochemical analysis of the selected isolate, Phoma herbarum R21 showed cellulase, pectinase, amylase, phosphatase and laccase activity, while it was negative for lipase, protease, chitinase and xylanase production compared to control (Table 3). Further, selected fungus effectively produces secondary metabolite siderophore, whereas, IAA and HCN production were not found. Phoma herbarum R21 which showed better pathogenicity towards weeds under study was chosen for further study.
Table 3: Production of enzymes and secondary metabolites by Phoma herbarum_R21
|
S.No. |
Enzymes and metabolites |
Phoma herbarum R21 |
S.No. |
Enzymes and metabolites |
Phoma herbarum R21 |
|
1 |
Cellulase |
+ |
7 |
Xylanase |
– |
|
2 |
Lipase |
– |
8 |
Pectinase |
+ |
|
3 |
Protease |
– |
9 |
Phosphate solubilization |
+ |
|
4 |
Amylase |
+ |
10 |
HCN production |
– |
|
5 |
Laccase |
+ |
11 |
Siderophore production |
+ |
|
6 |
Chitinase |
– |
12 |
IAA |
– |
(+) indicates enzyme/metabolite production, (-) indicates no enzyme/metabolite production
Effect of Phoma herbarum R21 on Pre-Germination and Post-Gemination Test of Weeds and Wheat.
The ability of fungal culture to inhibit seed germination and seedling growth of different weeds was examined by calculating inhibition percentage as compared to control. All controls were taken as 0% inhibition. Data in Table 4 and Fig. 1, demonstrate that Phoma herbarum reduced germination of weeds from 5% to 36 %. Maximum germination inhibition (36%) was observed in P. minor. About 27-59 % reduction in shoot length was observed in all the targeted weeds. Moreover, 29-61% reduction in root length and 41-72% reduction in vigour index was observed in all weeds, but C. album has showed overall maximum reduction in growth parameters (32-72%) by Phoma herbarum R21 compared to control.
Table 4: Phytotoxic effect of Phoma herbarum R21 on pre-germination treatment of target weeds.
|
Target weeds |
|
Germination percentage (%) |
Shoot length (cm) |
Root length (cm) |
Fresh weight total (mg) |
Vigour index |
|||||
|
Avena fatua |
Control |
95.0 ±4.08 |
8.5 ±0.23 |
8.7 ±0.15 |
135.3 |
1634 ±35.88 |
|||||
|
|
Treated |
90.0 ±4.08 |
4.6 ±0.15 |
6.1 ±0.30 |
70.6 |
963 ±24.25 |
|||||
|
|
GIP (%) |
5.26 |
45.88 |
29.89 |
47.85 |
41.06 |
|||||
|
Phalaris minor |
Control |
86.6 ±2.35 |
8.7 ±0.18 |
2.5 ±0.30 |
4.8 ±0.13 |
970.6 ±10.63 |
|||||
|
|
Treated |
55.0 ±4.08 |
6.2 ±0.30 |
1.5 ±0.10 |
1.1 ±0.03 |
421.3 ±10.60 |
|||||
|
|
GIP (%) |
36.05 |
29.14 |
40.41 |
77.29 |
56.59 |
|||||
|
Chenopodium album |
Control |
91.6 ±2.35 |
3.7 ±0.14 |
3.5 ±0.25 |
7.3 ±0.15 |
655.4 ±36.27 |
|||||
|
|
Treated |
63.3 ±2.36 |
1.6 ±0.18 |
1.3 ±0.35 |
3.6 ±0.26 |
169.1 ±27.40 |
|||||
|
|
GIP (%) |
32.26 |
57.84 |
61.30 |
51.23 |
72.02 |
|||||
GIP- Growth Inhibition Percentage (%), *Controls are taken as 0% inhibition.
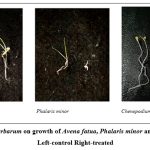 |
Figure 1: Effect of P. herbarum on growth of Avena fatua, Phalaris minor and Chenopodium album. Left-control Right-treated |
Phoma herbarum is first reported for showing significant growth promoting activity in wheat seedlings. 102 – 188% increase in different growth parameters (shoot length, root length, biomass, and vigour index) was observed (Table 5 and Fig. 2). This signifies that Phoma herbarum is selectively affecting weeds and not the wheat crop and hence is helpful in early-stage removal of weeds especially A. fatua and P. minor that mimic wheat phenotypically.
Table 5: Plant growth promoting potential of Phoma herbarum on the growth of wheat.
|
Wheat varieties |
|
Germination percentage (%) |
Shoot length (cm) |
Root length (cm) |
Fresh weight total (mg) |
Vigour index |
||||
|
Poshan |
Control |
98.3 ±2.36 |
7 ±0.25 |
8.1 ±0.28 |
48.5 ±0.05 |
1481.8 ±40.43 |
||||
|
Treated |
98.3 ±2.36 |
9.5 ±0.15 |
11.5 ±0.32 |
91.3 ±0.85 |
2066.9 ±10.05 |
|||||
|
GP (%) |
100 |
136.4 |
142.3 |
188.4 |
139.5 |
|||||
|
Tejas |
Control |
96.6 ±2.36 |
8.1 ±0.19 |
9.1 ±0.14 |
89.5 ±0.25 |
1665.5 ±30.88 |
||||
|
Treated |
98.3 ±2.36 |
10.7 ±0.35 |
15.5 ±0.32 |
112.8 ±0.60 |
2570.4 ±27.40 |
|||||
|
GP% |
102 |
131.4 |
169.79 |
125.9 |
154.3 |
|||||
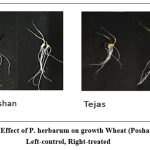 |
Figure 2: Effect of Phoma herbarum on growth Wheat (Poshan and Tejas). Left-control, Right-treated. |
GP- Growth Percentage. *Controls are taken as 100 % growth.
The ability of fungus to selectively infect weeds over wheat was examined by detached leaf assay (post-germination test). This test revealed the selective phytotoxic potential of Phoma herbarum against targeted weeds. Hence, Phoma herbarum can also be applied on crop after germination.
Effect of Phoma herbarum R21 CFCF on Pre-Germination and Post-Gemination Test of Weeds and Wheat.
The fungal cultural filtrate was tested on weeds and different growth parameters were evaluated after treatment (Table 6). Out of all the treatments, 14th day CFCF of Phoma herbarum was effectively inhibiting germination by 63%, 47% and 40% in C. album, A. fatua, and P. minor respectively. 93% reduction in growth parameters were observed upon CFCF treatment. Following, the 11th day CFCF treatment showed highest inhibition of shoot and root length on C. album followed by A. fatua and P. minor, resulted in a 63 % decrease in plant biomass.
Effect of CFCF was also reported after evaluating different growth parameters on Poshan and Tejas varieties of durum wheat (Table 6). Among various treatments, on 8th day CFCF has no effect on germination of wheat. Whereas, 11th and 14th day CFCF has inhibited wheat germination by 11-37 %. 8th day CFCF has less effect on growth of Poshan and Tejas in contrast to 14th day CFCF which has shown maximum effect on growth of durum wheat. This data suggested that CFCF cannot be used pre-germination for control of weeds in wheat field.
Table 6: Effect of CFCF on growth of A. fatua, P. minor, and C. album.
|
Target weeds |
|
Germination percentage (%) |
Shoot length (cm) |
Root length (cm) |
Fresh weight total (mg) |
Vigour index |
|
Avena fatua |
Control |
95.0 ±2.36 |
4.5 ± 0.30 |
5.2 ±0.15 |
59.8 ±3.17 |
965.0 ±15 |
|
T1 |
76.6 ±4.71 |
3.5 ±0.11 |
2.5 ±0.20 |
42.5 ±2.5 |
459.6 ±38 |
|
|
T2 |
46.7 ±2.36 |
1.0 ±0.21 |
0.7±0.10 |
26.2 ±2.2 |
79.3 ±20 |
|
|
T3 |
46.7 ±2.36 |
1.2 ±0.10 |
0.7±0.05 |
33.1 ±0.00 |
88.73 ±11 |
|
|
Phalaris minor |
Control |
86.6 ±2.36 |
5.5 ±0.30 |
2.0 ±0.15 |
4.0 ±0.00 |
649.5 ±10 |
|
T1 |
65.0 ±4.08 |
3.2 ±0.10 |
1.3 ±0.25 |
1.5 ±0.25 |
276.3 ±3.30 |
|
|
T2 |
55.0 ±4.08 |
2.6 ±0.30 |
1.0 ±0.02 |
1.0 ±0.02 |
199.1 ±2.90 |
|
|
T3 |
51.6 ±2.36 |
2.4 ±0.20 |
0.8 ±0.22 |
1.0 ±0.13 |
166.2 ±3.26 |
|
|
Chenopodium album |
Control |
91.6 ±2.36 |
3.7 ±0.33 |
3.6 ±0.05 |
7.2 ±0.35 |
649.8 ±0.44 |
|
T1 |
56.6 ±2.36 |
0.6 ±0.64 |
0.4 ±0.04 |
3.5 ±0.50 |
54.1 ±3.48 |
|
|
T2 |
63.3 ±4.08 |
0.5 ±0.03 |
0.2 ±0.03 |
2.4 ±0.20 |
44.3 ±2.31 |
|
|
T3 |
33.3 ±4.08 |
0.6 ±0.03 |
0.3 ±0.03 |
4.3 ±0.25 |
28.6 ±2.00 |
|
|
Wheat Varieties |
||||||
|
Poshan |
Control |
100 ±0.00 |
4.3 ±0.20 |
7.5 ±0.16 |
54.3 ±1.70 |
1175.5 ±4.50 |
|
T1 |
100 ±0.00 |
1.4 ±0.09 |
5.4 ±0.25 |
29.5 ±0.00 |
684.5 ±15.5 |
|
|
T2 |
63.5 ±2.36 |
1.4 ±0.02 |
2.4 ±0.18 |
26.7 ±0.68 |
237.6 ±18.78 |
|
|
T3 |
68.3±2.36 |
1.6 ±0.05 |
2.3 ±0.26 |
25.8 ±0.28 |
268.1 ±8.84 |
|
|
Tejas |
Control |
100 ±0.00 |
3.32 ±0.24 |
10.6 ±0.35 |
63.4 ±0.18 |
1397 ±11.0 |
|
T1 |
100 ±0.00 |
2.76 ±0.10 |
7.7 ±0.06 |
51.3 ±0.35 |
1050 ±4.0 |
|
|
T2 |
86.6 ±4.71 |
1.3 ±0.21 |
5.9 ±0.20 |
42.5 ± 2.35 |
622.9 ±23.0 |
|
|
T3 |
88.3 ±2.36 |
1.7 ±0.21 |
5.0 ±0.12 |
43.8 ±1.69 |
595.7 ±39.7 |
T= Treatment, T1 = 8th day CFCF, T2 = 11th day CFCF, T3 = 14th day CFCF.
Post-germination effect of CFCF was recorded by performing detached leaf assay. Which indicates the selective phytotoxicity against weeds over wheat in 7 days. Hence, CFCF is safe to use in wheat field for weed control once the seedling appeared.
Antifungal activity of Phoma herbarum R21
In dual plate assay, Phoma herbarum inhibited and controlled the radial mycelial growth of phytopathogen (Fig. 3).
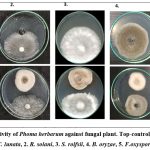 |
Figure 3: Antagonistic activity of Phoma herbarum against fungal plant. Top-control, bottom-dual plate assay. 1. C. lunata, 2. R. solani, 3. S. rolfsii, 4. B. oryzae, 5. F.oxysporium |
The percentage of inhibition of radial growth (PIRG) values ranged from 31.96 to 57.73% (Fig. 4). The highest PIRG values 57.73% and 51.98% were observed with C. lunata and R. Solani. The lowest inhibition (30.5%) was observed with B. oryzae.
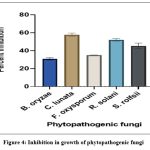 |
Figure 4: Inhibition in growth of phytopathogenic fungi |
Discussion
Over 1.8 tonnes of chemical weedicide are consumed annually in Madhya Pradesh (India), which adversely affect the environment (ICAR, Directorate of Weed Research, 2016). Chemical herbicides depict a profound harmful effect on biotic components of environment, and also contribute to emergence of resistant weed populations24. Despite the recognition of these negative impacts, few bioherbicides have been registered globally25,26. However, to minimize the toxicity in food chain and promote organic wheat farming, it is crucial to develop potential bioherbicides and prioritize the adoption of biological weed control methods in almost all agrarian countries especially in India.
In this study, potential fungal isolate DGL 8C identified as Phoma herbarum R21 Accession No. (ON705696). Phoma herbarum R21 showed pathogenesis on the detached leaves of target weeds such as A. fatua, C. album and P. minor. Pre-germination treatment of target weed seeds using Phoma herbarum R21 showed significant inhibition from 5 to 77%. Among the tested weed species, C. album and P. minor showed the highest level of inhibition as compared to A. fatua. The pre-germination treatment of CFCF revealed its effectiveness in inhibiting growth of weed upto 95% but inhibiting wheat growth upto 65%. This inhibition might be because of presence of some phytotoxic chemicals in CFCF. This data suggested that fungal spores are more suitable for pre-germination application against weeds in wheat field as compared to CFCF.
Phoma herbarum strains and their metabolites have been reported in literature as a biocontrol agent aginst weeds such as Taraxacum officinale, Trianthema portulacastrum, Parthenium hysterophorus, Lantana camera, Xanthium strumarium, Cassia tora, Hyptis suaveolens, Sida acuta, Antignon leptopus, Amaranthus hypochondriactus27,28,29,30,31,32. Phoma herbarum was also reported as an effective biocontrol agent against glyphosate resistant E. indica33.
A bioherbicide candidate must overcome the plant defence mechanisms of target weed plant and establish a compatible relationship34. Virulence factors, such as cell wall degrading enzymes and phytotoxic secondary metabolites, play a crucial role in facilitating entry and disrupting the plant’s metabolism35,36,7,37. The production of hydrolytic enzymes and secondary metabolites, including cellulase, pectinase, laccase and amylase activities indicate the pathogenicity of Phoma herbarum, along with this it also exhibits growth-promoting abilities through the production of phosphatase and siderophore. The hydrolytic enzymes take part in successful infection to weeds35 whereas, the plant growth promoting traits such as phosphatase and siderophore activity can take part in wheat growth promotion.
Along with strong bioherbicidal properties in pre-germination test, Phoma herbarum also promotes growth of wheat varieties (Poshan and Tejas) upto 88%. To the best of our knowledge, after a comprehensive review of the available literature, this study presents the first report of growth promotion in wheat using Phoma herbarum.
Total of 22% loss in wheat yield was recorded in 2019 due to fungal diseases38. Hence, the antifungal property of Phoma herbarum against five phytopathogenic fungi of wheat (Rhizoctonia solani, Sclerotium rolfsii, Bipolaris oryzae, Curvularia lunata and Fusarium oxysporum) is a benefit to wheat crop protection.
The findings of this study have highlighted the multiple actions of Phoma herbarum and provide a practical basis for further application in field for biocontrol of weeds and wheat growth promotion. Further research, suitable formulation and integration of other weed management strategies shall help in making it as a promising candidate for eco-friendly weed control approach.
Conclusion
Phoma herbarum R21, selected from forty-three isolates, exhibits bioherbicidal activity against three targeted weeds. The fungus and CFCF both show growth supressing characteristics on weeds. Current study recommended application of fungus in both pre-germination and post-germination stages for effective inhibition of weeds. While, CFCF can selectively inhibit weeds in post-germination stage. This fungus has been reported as a producer of bioherbicidal metabolites. In addition to bioherbicidal activity, Phoma herbarum has growth promotion activity on wheat and antifungal activity against potential phytopathogenic fungi.
This triple action promotes the application of Phoma herbarum in agricultural land with suitable formulation. Thus, Phoma herbarum R21 is suggested as a potential bioherbicide candidate to control several weeds and promote wheat growth. It would be valuable to investigate the capabilities of Phoma herbarum R21 under natural conditions, both microbial application in soil as well as CFCF application at various growth stages.
Acknowledgement
This work is supported by UGC- NET JRF fellowship program. The authors acknowledged the facility of the School of Biotechnology, DAVV. Indore and School of Biological Sciences, Networking Resource Centre in Biological Sciences (NRCBS) for the NRCBS-UGC visiting fellowship program, Madurai Kamaraj University, Madurai, Tamil Nadu, India.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal, subjects, or any material that requires the ethical approval.
Informed Consent Statement
All authors have given their consent for publication in “Current Agriculture Research Journal”.
Authors’ Contribution
N.G. (Neha Gupta): Carried out the experiment, designed and wrote the manuscript.
B.R. (Baishali Roy): Wrote the manuscript.
A.N. (Anand Nighojkar) (corresponding author): Designed the experiment, analysis of experiment, interpretation of result and reviewed the manuscript.
V.S. (Vellasamy Shanmugaiah): Designed the work and analysed the data.
All authors read and approved the final manuscript.
References
- Gharde, SinghP.K., Dubey R.P., Gupta P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018;107:12-18. DOI:10.1016/j.cropro.2018.01.007.
CrossRef - Savita, SharmaA. In: Giri , Prasad R., Wu Q.S., Varma A., eds. Biofertilizers for Sustainable Agriculture and Environment. Soil Biology. 2019;55:395-411. DOI:10.1007/978-3-030-18933-4_18.
CrossRef - Shanmugaiah V., Ramesh S., Jayaprakashvel M., Mathivanan N. Biocontrol and plant growth promoting potential of Pseudomonas sp. MML2212 from the rice rhizosphere. Mitt-biol Bundesanst land Forstwirtsch. 2006;408:320.
- Currie A.F., Hinz H.L., Shaw D.R., Bhatti M.A., Neupane A. Bioherbicides: opportunities and challenges for weed management. Weed Sci. 2020;68:3-13.
- Dutta W., Ray P. A glimpse into the compatibilities and conflicts between arthropods and fungal biological control agents of aquatic weed waterhyacinth. Phytoparasitica. 2017;45(3):429-437. DOI:10.1007/s12600-017-0605-y.
CrossRef - Hamayun M., Khan S.A., Khan A.L., et al. Phoma herbarum as new gibberellin producing and plant growth promoting fungus. J Microbiol Biotechnol. 2009;19(10):1244-1249. DOI:10.4014/jmb.0901.030.
- Harding M.W., Raizada M.N. Controlling weeds with fungi, bacteria and viruses: a review. Front Plant Sci. 2015;6:1-9. DOI:10.3389/fpls.2015.00659.
CrossRef - Kalam S., Pandey A., Pandey A.K. Mass production and formulation of herbicidal metabolites from Phoma herbarum FGCC # 54 for management of some prominent weeds of Central India. AIJRFANS. 2014;5(1):40-47.
- Cimmino A., Andolfi A., Zonno M.C., et al. Chenopodolin: A phytotoxic unrearranged ent-pimaradiene diterpene produced by Phoma chenopodicola, a fungal pathogen for Chenopodium album J Nat Prod. 2013;76(7):1291-1297. DOI:10.1021/np400218z
CrossRef - Bailey K.L., Pitt W.M., Falk S., Derby J. The effects of Phoma macrostoma on nontarget plant and target weed species. Biol Control. 2011;58(3):379-386. DOI:10.1016/j.biocontrol.2011.06.001.
CrossRef - Chiang M.Y., Van Dyke C.G., Leonard K.J. Evaluation of endemic foliar fungi for potential biological control of Johnsongrass (Sorghum halepense): screening and host range tests. Plant Dis. 1989;73(6):459-464.
CrossRef - Kiiskinen L.L., Rättö M., Kruus K. Screening for novel laccase-producing microbes. J Appl Microbiol. 2004;97(3):640-646. DOI:10.1111/j.1365-2672.2004.02348.x.
CrossRef - Ferrari A.R., Gaber Y., Fraaije M.W. A fast, sensitive and easy colorimetric assay for chitinase and cellulase activity detection. Biotechnol Biofuels. 2014;7(1):37. DOI:10.1186/1754-6834-7-37.
CrossRef - Pikovskaya R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya. 1948;17:362-370.
- Beg Q.K., Bhushan B., Kapoor M., Hoondal G.S. Production and characterization of thermostable xylanase and pectinase from Streptomyces sp QG-11-3. J Ind Microbiol Biotechnol. 2000;24(6):396-402. DOI:10.1038/sj.jim.7000010.
CrossRef - Lorck H. Production of hydrocyanic acid by bacteria. Physiol Plant. 1948;1(2):142-146. DOI:10.1111/j.1399-3054.1948.tb07118.x.
CrossRef - Alexander D.B., Zuberer D.A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fert Soils. 1991;12(1):39-45. DOI:10.1007/BF00369386.
CrossRef - Tsavkelova E.A., Cherdyntseva T.A., Botina S.G., Netrusov A.I. Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res. 2007;162(1):69-76. DOI:10.1016/j.micres. 2006.07.014.
CrossRef - White T.J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications Academic Press; 1990:315-322
CrossRef - Saxena S., Pandey A.K. Evaluation of an indigenous isolate of Alternaria alternata (LC# 508) for use as a mycoherbicide for Lantana camara L. Crop Prot. 2002;21(1):71-73. DOI:10.1016/S0261-2194(01)00042-4.
CrossRef - Mohammed Y.M.M., Badawy M.E.I. Potential of phytopathogenic fungal isolates as a biocontrol agent against some weeds. Egypt J Biol Pest Control. 2020;30(1):1-9. DOI:10.1186/s41938-020-00295-0.
CrossRef - Karmegham N., Vellasamy S., Natesan B., Sharma M.P., Al Farraj D.A., Elshikh M.S. Characterization of antifungal metabolite phenazine from rice rhizosphere fluorescent pseudomonads (FPs) and their effect on sheath blight of rice. Saudi J Biol Sci. 2020;27(12):3313-3326.
CrossRef - Skidmore A.M., Dickinson C.H. Interactions between germinating spores of Septoria nodorum and phylloplane fungi. Trans Br Mycol Soc. 1976;66:45-56.
CrossRef - Schütte G., Eckerstorfer M., Rastelli V., et al. Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ Sci Eur. 2017;29(1):5. DOI:10.1186/s12302-016-0100-y.
CrossRef - Morin L. Progress in biological control of weeds with plant pathogens. Annu Rev Phytopathol. 2020;58:201-223. DOI:10.1146/annurev-phyto-010820-012823.
CrossRef - Hasan M., Ahmad-Hamdani M.S., Rosli A.M., Hamdan H. Bioherbicides: an eco-friendly tool for sustainable weed management. Plants (Basel). 2021;10(6):1212. DOI:10.3390/plants10061212.
CrossRef - Fausto Rivero-Cruz J., García-Aguirre G., Cerda-García-Rojas C.M., Mata R. Conformational behavior and ansolute stereostructure of two phytotoxic nonenolides from the fungus Phoma herbarum. Tetrahedron. 2000;56(30):5337-5344. DOI:10.1016/S0040-4020(00)00469-5.
CrossRef - Neumann S., Boland G.J. Influence of host and pathogen variables on the efficacy of Phoma herbarum, a potential biological control agent of Taraxacum officinale. Can J Bot. 2002;80(4):425-429. DOI:10.1139/b02-024
CrossRef - Schnick P.J., Boland G.J. 2,4-D and Phoma herbarum to control dandelion (Taraxacum officinale). Weed Sci. 2004;52(5):808-814. DOI:10.1614/WS-03-085R.
CrossRef - Vikrant P., Verma K.K., Rajak R.C., Pandey A.K. Characterization of a phytotoxin from Phoma herbarum for management of Parthenium hysterophorus Phytol Pathol. 2006;154(7-8):461-468. DOI:10.1111/j.1439-0434.2006.01129.x.
CrossRef - Ray P., Vijayachandran L.S. Evaluation of indigenous fungal pathogens from horse purslane (Trianthema portulacastrum) for their relative virulence and host range assessments to select a potential mycoherbicidal agent. Weed Sci. 2013;61(4):580-585. DOI:1614/WS-D-12-00076.1
CrossRef - Singh A.K., Pandey A.K. Screening of herbicidal potential of crude extract of isolated fungal strains against some noxious weeds of India: a preliminary evaluation. J Agron Agric Sci. 2019;2:015
CrossRef - (Rusli et al. 2021). Rusli M.H. Shariffah muzaimah S.A.1., Maizatul S.M. and Idris A.S. Effects of Phoma herbarum as a biological control agent of glyphosate resistant Eleusine indica. J Oil Palm Res. 2022;34(3):465-474.
CrossRef
- Trognitz F., Hackl E., Widhalm S., Sessitsch A. The role of plant–microbiome interactions in weed establishment and control. FEMS Microbiol Ecol. 2016;92(10). DOI:10.1093/femsec/fiw138.
CrossRef - Ghorbani R., Leifert C., Seel W. Biological control of weeds with antagonistic plant pathogens. Adv Agron. 2005;86:191-225. DOI:10.1016/S0065-2113(05)86004-3.
CrossRef - Stergiopoulos I., Collemare J., Mehrabi R., De Wit P.J. Phytotoxic secondary metabolites and peptides produced by plant pathogenic Dothideomycete fungi. FEMS Microbiol Rev. 2013;37(1):67-93. DOI:10.1111/j.1574-6976.2012.00349.x.
CrossRef - Cordeau S., Triolet M., Wayman S., Steinberg C., Guillemin J. Bioherbicides: dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016;87:44-49. DOI:10.1016/j.cropro.2016.04.016.
CrossRef - Kayim M., Nawaz H., Alsalmo A. Fungal Diseases of Wheat. IntechOpen; 2022. DOI:10.5772/intechopen.102661.
CrossRef

