Introduction
The occurrence of this condition can be attributed to a soilborne fungus known as Fusarium oxysporum f. sp. vasinfectum. This disease causes significant yield losses and affects the economy of cotton farmers. Common controlling strategies for Fusarium wilt disease of cotton include soil conditioning, resistant varieties, crop rotation, and the use of chemical pesticides.1 Several fungicides are available for controlling Fusarium wilt pathogen, but they are expensive and have limited effectiveness. Several investigators reported use of different chemical fungicides for management of Fusarium wilt of cotton such as Pyraclostrobin, Mitiram, Captan, hexaconazole,2 Carbendazim,3,4 Copper oxychloride,4 Dithane M-45 Mancozeb, and Thiophanate methyl.5
But the application of chemical fungicides imposes drastic problems like affecting the beneficial microbes in soil, the development of resistance among pathogens against fungicides, pollution of the environment, and health problems. As Fusarium wilt poses a significant risk to cotton production. it is necessary to look for alternative methods of disease control that do not involve the use of harmful chemicals.
Over the last few years, there has been a growing emphasis on developing alternative and sustainable methods to effectively control plant diseases. These methods often focus on preventing diseases from occurring in the first place, rather than relying on pesticides to control them. Using rhizospheric microorganisms to control plant pathogens is a promising alternative to chemical methods. Rhizobacteria are naturally occurring bacteria that can be found in the soil surrounding plant roots. Some rhizobacteria exhibit plant growth-promoting (PGPR) and biocontrol properties, making them promising candidates for managing plant diseases. Researchers identified different microorganisms such as Bacillus sp., 6,7Pseudomonas sp.,8-10 Serratia sp.,8,11 and Trichoderma sp.,12-15 which would be potential candidates for management of plant diseases instead of chemical fungicides.
There is limited literature on controlling Fusarium wilt in Bt-cotton using microorganisms. Despite the work of many researchers in the field of biological control, previous studies have mainly explored the use of endophytic bacteria16-17 and Trichoderma sp. 18-19 for managing Fusarium wilt in cotton.
In present research, our prime goals were: i) to test the antifungal activity of a rhizobacterial isolate against the Fusarium wilt pathogen by in vitro screening. ii) to understand the microbial control mechanism of the efficient rhizobacterial isolate by examining the production of diffusible and volatile organic compounds, siderophore, chitinase enzyme protease enzyme, and phosphate solubilization.
Fusarium wilt Phytopathogen of Bt-Cotton
Fusarium wilt phytopathogen of Bt-cotton which was previously isolated in research work conducted in the Department of Microbiology by freshly activating on PDA agar plate.
Rhizobacterial Bacterial isolate
Rhizobacterial isolate RLS76, which was isolated in an earlier study and had antifungal potential against Alternaria sp. was used to study the antifungal activity against a fungal pathogen, Fusarium oxysporum f. sp. vasinfectum. by activating on freshly prepared nutrient agar.
In Vitro Antifungal Activity
The dual culture technique was employed to assess the antifungal efficacy of rhizobacteria isolate RLS76 against the Fusarium wilt pathogen of Bt cotton.20 Petri dishes were prepared with potato dextrose agar (PDA). To ensure a consistent separation between the phytopathogen and the antagonist, a 5 mm fungal disc from a 5-day-old culture and a 24-hour-old culture of RLS76 rhizobacteria isolate were both inoculated 1 cm away from the center of the plate. This method was employed to maintain a constant distance between the two organisms. Additionally, a control plate was inoculated with only fungal culture. The plates were left to incubate at room temperature for a duration of 6 days. The antifungal activity of the rhizobacteria isolate was evaluated by determining the percentage of inhibition in radial growth.21
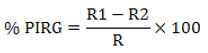
Where, R1 refers to the radius of the fungal colony on the control plate, while R2 refers to the radius of the fungal colony towards the antagonist colony on test pate.
Identification of Biocontrol Agent
The rhizobacterial isolate RLS76, showing antifungal activity by inhibiting the radial mycelial growth of the fusarium wilt pathogen of Bt cotton, was identified by using 16S rRNA partial sequencing at ARI Pune, Maharashtra. The genomic DNA of RLS76 was extracted using Sigma’s “GenElute Bacterial Genomic DNA” Kit. polymerase chain reaction was conducted using the RPP2 (CCAAGCTTCTAGACGGITACCTTGTTA CGACTT) and FDD2 (CCGGATCCGTCG ACAGAGTTTG ATCITGGCTCAG) universal primers to amplify a 1.5 kb fragment for eubacteria. 22-23 The polymerase chain reaction products obtained from the previous reactions were then subjected to the cycle sequencing reaction, using only the SRV3-1 primer. After the reaction, the samples were purified and loaded onto the Avant 3100 Gene Analyzer for sequencing.
Mechanism of Biocontrol Agent
The antifungal activity of biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 was investigated by examining its ability to produce volatile compounds, diffusible compounds, siderophore, chitinase enzyme, protease enzyme, and phosphate solubilizing ability.
Recognition of Extracellular Diffusible Organic Compounds
The agar well diffusion assay was used to detect diffusible organic compounds.24 The biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 crude extract was obtained by culturing in Kings B broth at normal laboratory temperature on a shaking incubator, operating at 150 rpm, for a duration of 36-48 hours. Afterwards, three wells were formed on each of the Kings B plates using a sterilized Cork borer that had a diameter of 10 mm. These wells were subsequently labeled, and the crude extract was carefully loaded into each individual well. Following this, a fungal disc with a diameter of 5 mm, obtained from a fresh culture of F. oxysporum f. sp. vasinfectum was positioned at the center of the agar plate. The broth culture previously inoculated with Bacillus subtilis subsp. inaquosorum RLS76 was subjected to centrifugation at 8000 rpm and subsequently filtered using a 0.22 µ (Hi-media) syringe filter. This process was carried out to prepare the crude extract for testing the diffusible organic compounds. The well was individually filled with 100 µl of the crude extract in an aseptic condition, and control was maintained without the inoculation of crude extract. The control and test plate were incubated for 5 to 6 days at room temperature. Following the incubation period, the plates were carefully examined for inhibitory activity and the percentage of inhibition was calculated as described by Whipps.25
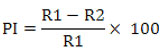
Where, R1 refers to the radius of the fungal colony on the control plate, while R2 refers to the radius of the fungal colony towards the antagonist colony on test plate.
Recognition of Extracellular Volatile Organic Compounds
The extracellular volatile organic compounds of the biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 was tested by using double plate method.20 In this method, a kings-B plate was inoculated with a 5 mm fungal disc of phytopathogen at the center, while the rhizobacterial isolate was inoculated at the center of a 90 mm agar plate individually. The bases of the two plates were then sealed together using adhesive tape. Additionally, a control plate was maintained without adding the bacterial isolate. This process was repeated three times using the same cultures. All the plates were nurtured at normal laboratory temperature for six days. The volatile organic compounds production was confirmed by measuring the inhibition of Fov, and the inhibition percentage was calculated as described by Whipps.25
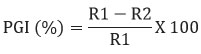
Where, R1 refers to the radius of the fungal colony on the control plate, while R2 refers to the radius of the fungal colony towards the antagonist colony on test plate.
Identification of Volatile Bioactive Compounds
The GCMS investigation of the crude extract of biocontrol agent B. subtilis subsp. inaquosorum RLS76-inoculated Kings B broth and nurtured at normal laboratory temperature for 3 days was conducted at IIT Bombay Powai, Mumbai. The analysis was performed using an Agilent-7890 machine equipped with an FID detector, Head Space injector, Combipal autosampler, and a Jeol Accu TOF GCV MS with a mass range of 10-2000 amu and mass resolution of 6000. A VF-5MS (5% phenyl methyl) capillary column measuring 30.0 m × 250 mm × 0.25 μm was utilized. The carrier gas used was helium at a rate of 1 µl/min. The column temperature was programmed to start at 60◦C and increase by 5 K/min until it reached 270◦C, where it was held isothermally for 38 min. The constituents were identified by comparing their mass spectral data with those present in the NIST library.
Siderophore Analysis
The qualitative and quantitative method was employed to conduct an analysis of siderophore production of biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76.
CAS Agar Plate Method for Siderophore
The universal Chrome Azurol S Plate method 26 was utilized to perform qualitative testing of siderophore in B. subtilis subsp. inaquosorum RLS76. In order to conduct the qualitative detection of siderophores, Petri plates were prepared by using 30 ml of CAS blue agar and allowing it to solidify. The 24-hour-old rhizobacterial isolate Bacillus subtilis subsp. inaquosorum RLS76 was then CAS agar plate was inoculated at its central region. Afterward, the plate was placed in a controlled environment at ambient temperature for a duration of fourteen days. The presence of siderophores was demonstrated by observing a color transformation of the CAS agar medium, which changed from blue to either purple or orange.
CAS Liquid Assay for Siderophore
The investigation of siderophore production was conducted using a modified succinate broth, as per the procedure described by Meyer and Abdallah.27 The medium was composed of the following components per liter: succinic acid (4g), K2HPO4 (6g), KH2PO4 (3g), (NH4)2 SO4 (1g), MgSO4 (0.2g), and pH adjusted to 7.0. To start the experiment, a 0.1 ml culture of Bacillus subtilis subsp. inaquosorum RLS76 was introduced into a 250 ml Erlenmeyer flask containing the Succinate medium. The flask was then placed on a shaking incubator and incubated at laboratory temperature for a duration of 24-72 hours. Throughout the incubation period, a 1 ml sample of the broth culture was collected at specific time intervals (e.g., 24, 36, 48, 60, 72 hours). Subsequently, the culture was subjected to centrifugation at 10,000 rpm in a cooling centrifuge set at 4°C for 10 minutes to obtain the supernatant.
The quantification of siderophore production was carried out by combining 0.5 ml solution of CAS with 0.5 ml of centrifuged culture filtrate. To set up a control, a sterile succinate broth was used instead of centrifuged culture filtrate. The OD of the test and control samples determined at 630 nm, and the percentage of SU was calculated. 28
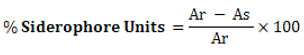
Where,
Ar = Absorbance of reference and As = Absorbance of test sample at 630 nm.
Chitinase Enzyme Plate Assay
The determination of chitinase enzyme production by the biocontrol agent B. subtilis subsp. inaquosorum RLS76 was conducted using colloidal chitin agar supplemented with 0.4% colloidal chitin.29 The bacterial suspension was used to inoculate the chitin agar plate, which was then left to incubate at room temperature for a period of 4 days. Subsequently, the plates were inspected to figure out if there was a chitin hydrolysis zone surrounding the culture that was spot inoculated.
Protease Enzyme Plate Assay
The assessment of protease enzyme production was conducted by introducing the biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 onto a skim-milk agar plate.30 The plate was inoculated with the rhizobacterial isolate and placed in an incubator at room temperature for a period of 24-48 hours. Following the incubation, the presence of a clear halo zone around the rhizobacterial colony on the skim milk agar plate was observed, and its measurement in millimeters was recorded.
Phosphate Solubilization Assay
The phosphate solubilization ability of the biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 was evaluated using NBRIP agar plate, as described by Nautiyal.31 The rhizobacterial isolate was then spot inoculated on the NBRIP agar plate and incubated at normal laboratory temperature for 5 days. A clear halo around the colony was identified as phosphate solubilizers.
Results and Discussion
In Vitro Antifungal Activity
The rhizobacterial isolate RLS76 was found to reduce the fungal growth of the phytopathogen F. oxysporum f. sp. vasinfectum whose inhibition percentage was recorded as 85.39 % (Photo Plate 1 and Table 1).
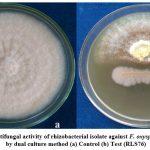 |
Plate 1: Screening of antifungal activity of rhizobacterial isolate against F. oxysporum f. sp. vasinfectum by dual culture method (a) Control (b) Test (RLS76) |
Table 1: Antifungal activity of rhizobacterial isolate against F. oxysporum f. sp. vasinfectum
| Sr. No. | Rhizobacterial isolate | % of inhibition of radial growth of Fov |
| 1 | RLS76 | 85.39 |
A number of scientists extracted the rhizobacterial bacterial isolates from the disease-free cotton field and examined their antifungal properties against the fungal pathogen Fov using the dual culture method. Among the nine Bacillus strains obtained from the rhizosphere of cotton plants, two strains, BP-9 and BP-13 displayed the highest percentage of killing growth of the fungal pathogen F. oxysporum f. sp. vasinfectum, with values of 62.50% and 51.25% respectively.32 The rhizospheric isolate belonging to Pseudomonas spp. MRh 42 inhibited 66.60% growth of Fov a phytopathogen of fusarium wilt disease, in cotton crops.33 In the same vein, Saccharothrix algeriensis NRRL B-24137 exhibited a noteworthy antifungal capacity against the Fusarium wilt phytopathogen of cotton in laboratory conditions. It effectively suppressed 68% of the mycelial growth of the pathogen, surpassing the control group.3 Furthermore, Bacillus subtilis, a naturally occurring bacterial antagonist, demonstrated a significant inhibitory effect of 47.29% on the growth of the fungal pathogen Fusarium oxysporum f. sp. vasinfectum, when compared to control.2 Similarly, the strain of Bacillus spp Rz141 reduced the fungal growth of Fov4 by 52% compared to the control by the dual culture method.34
The highest antimycotic action documented against F. oxysporum f. sp. vasinfectum by earlier researchers ranged from 47.29 to 68.00 %. However, in the present research work, rhizobacterial isolate RLS76 recorded an 85.39 % reduction in the fungal growth of Fusarium oxysporum f. sp. vasinfectum. The research work conducted previously indicates that the rhizobacterial isolate RLS76 has shown remarkable efficacy in managing the Fusarium wilt disease of Bt cotton.
Identification of Biocontrol Agent
The computer software (Applied Biosystems) DNA Sequence Analyzer was used to analyze the partial sequence of the RLS76 isolate’s 16S ribosomal RNA gene. The sequence was subjected to comparison with entries in the NCBI GenBank using the blast algorithm, which resulted in the identification of its closest phylogenetic affiliation as 99.53% with Bacillus subtilis subsp. inaquosorum RLS76. (Fig. 1).
 |
Figure 1: Phylogenetic tree of Bacillus subtilis subsp. inaquosorum RLS76. |
Mechanism of Biocontrol Agent
Recognition of Extracellular Diffusible Organic Compounds
The crude extract of biocontrol agent Bacillus subtilis subsp. immunoserum RLS76 showed reduction in fungal growth of 34.38% by producing diffusible non-volatile antifungal organic compounds (Table 2).
Table 2: Production of antifungal extracellular diffusible and volatile organic compound by biocontrol agent against the fungal plant pathogen.
| Sr No. | Biocontrol agent | Metabolites | Percentage of Inhibition Fusarium oxysporum f. sp. vasinfectum |
| 1 | Bacillus subtilis subsp. inaquosorum RLS76 | Extracellular diffusible organic compounds produced by agar well diffusion assay | 34.38 |
| Extracellular volatile organic compound by double plate assay | 54.84 |
The potential of Bacillus sp. to inhibit pathogenic fungi is principally based on their production of a variable variety of diffusible non-volatile organic compounds with antimicrobial properties.35 The B. subtilis GM2 strain harbors four genes responsible for encoding antimicrobial peptides, namely iturin, bacillomycin, bacitracin, and surfactin. Conversely, the GM5 strain possesses only two genes that encode the nearly same peptides, specifically surfactin and fengycin.36 B. amyloliquefaciens DHA55 and Bacillus amyloliquefaciens An6 produced multiple bioactive antifungal lipopeptide compounds belonging to iturin, surfactin, fengycin, and bacillomycin and showed significant antifungal activities against F. oxysporum.37-38 Some strains of Bacillus spp. S1301, S1967, S2535, and S2536 had an antifungal effect on the normal development of the Fusarium oxysporum f. sp. vasinfectum colony, which provided 12.92%, 27.31%, 33.06%, and 30.43% toxic effects respectively.39 The toxic effects were most frequent between the seventh and eighth days of the experiment as compared to the control. The rhizobacterial isolate isolated from tomato rhizosphere Bacillus cereus RFP63 showed the highest inhibitory impact (44.5%) on Fol after a week of incubation in the agar well diffusion method by secreting nonvolatile diffusible metabolites.40 The rhizobacterium Bacillus subtilis F62 reduced the mycelial growth of Fusarium sp., which varied from 35.4% to 63.6% measured on the 14th day of the experiment concerning the control.41
From above reports, it was clear that our biocontrol agent, Bacillus subtilis subsp. inaquosorum RLS76 showed 34.38% inhibitory activity, which was a lesser inhibitory effect than Russi et al. (2022)41 but produced some kinds of diffusible non-volatiles organic compounds that inhibited the growth of Fusarium oxysporum. Further analysis is needed for these diffusible organic compounds.
Recognition of Extracellular Volatile Organic Compounds
The biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 inhibited 54.84 fungal growth of Fov by producing extracellular volatile organic compounds in a closed environment (Table 2).
The biocontrol of phytopathogens has been found to be significantly influenced by the production of volatile secondary metabolites, including HCN 42 and ammonia, 43 by rhizobacterial bacteria such as Bacillus and Pseudomonas strains.44 The antagonistic bacterial strain of Bacillus Rz141 showed 24% inhibition of Fov4, a phytopathogen causing Fusarium wilt disease of Pima cotton, by producing volatile metabolites.34 Similarly, the two strains of Bacillus spp. S1823 and S2536 were evaluated for the production of volatile organic compounds and reduced the growth of Fov by 11.51% and 15.51% respectively, from the fifth day to the last day of the experiment as compared to the control.39 The Bacillus halotolerans strains RFP57 and RFP74 isolated from rhizobacterial soil of the tomato crop inhibited 67% and 34% fungal growth of the Fol respectively, in the double plate method by producing volatile metabolites.40
The research findings documented in different scientific journals revealed that our results with the rhizobacterial isolate Bacillus subtilis subsp. inaquosorum RLS76 against Fusarium oxysporum f. sp. vasinfectum are better than the results documented by earlier research workers by producing volatile secondary metabolites.
Identification of Volatile Bioactive Compounds
The chromatogram revealed 12 peaks during the GC-MS analysis of the crude extract obtained from the biocontrol agent, Bacillus subtilis subsp. inaquosorum RLS76. (Fig. 2). On the basis of spectral data, four compounds were recognized, namely Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-; Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl); 2,5-cyclohexadiene-1,4-dione, 2,5-Dihydroxy-3-methoxy-6-methyl- and 9,12-Octadecadienoic acid (Z,Z)-,phenylmethyl ester. Out of four compounds, three show antimicrobial activity except 2,5-Dihydroxy-3-methoxy-6-methyl (Table 3 and Fig. 3, 4, 5, 6 and 7).
 |
Figure 2: GC-MS Chromatogram of crude extract of biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 |
Table 3: Identification of volatile bioactive compounds in crude extract of biocontrol agent B. subtilis subsp. inaquosorum RLS76
| Retention time | Compound | Formula | Mol. Wt. g/mol | Similarity (%) | Biological activity | Reference |
| 16.75 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 154 | 72.5 | Anti-microbial Anti-oxidant | 45-46 |
| 17.43 and 19.59 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C11H18N2O2 | 210 | 46.9 | Anti-bacterial | 49,51 |
| 20.78 | 2,5-cyclohexadiene-1,4-dione, 2,5-Dihydroxy-3-methoxy-6-methyl- | C8H8O5 | 184 | 9.29 | Unknown | |
| 24.73 | 9,12-Octadecadienoic acid (Z,Z)-,phenylmethyl ester | C25H38O2 | 370 | 10.7 | Anti-microbial Anticancer | 69 |
The actinomycetes crude extract from a novel strain of Streptomyces sp. strain MUSC 149T displayed remarkable antioxidant activity. Furthermore, chemical analysis confirmed the existence of a volatile metabolite Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- by GCMS with antioxidant properties.45 Similarly, Bacillus tequilensis MSI45 a sponge associated bacterial strain whose crude extract showed the occurrence of pyrrolo[1,2-a]pyrazine-1,4-dione,hexahydro- a bioactive antimicrobial secondary metabolite.46
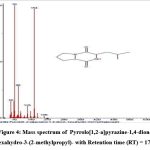 |
Figure 3: Mass spectrum of Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- with Retention time (RT) = 16.75 |
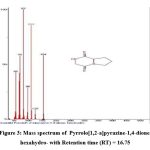 |
Figure 4: Mass spectrum of Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- with Retention time (RT) = 17.43 |
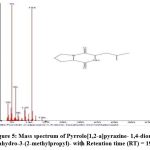 |
Figure 5: Mass spectrum of Pyrrolo[1,2-a]pyrazine- 1,4-dione, hexahydro-3-(2-methylpropyl)- with Retention time (RT) = 19.59 |
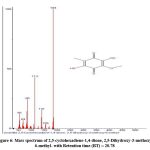 |
Figure 6: Mass spectrum of 2,5-cyclohexadiene-1,4-dione, 2,5-Dihydroxy-3-methoxy-6-methyl- with Retention time (RT) = 20.78 |
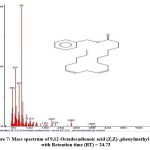 |
Figure 7: Mass spectrum of 9,12-Octadecadienoic acid (Z,Z)-,phenylmethyl ester with Retention time (RT) = 24.73. |
The partial characterization of the ethyl-acetate extracts of Bacillus pumilus MMM,47 Bacillus amyloliquefaciens subsp. amyloliquefaciens 48 and three strains of Bacillus sp. obtained from wild bees honey49 was performed using the GCMS and identified Pyrrolo [1,2-a] pyrazine-1,4-dion, hexahydro-3-(2-methylpropyl)- the main bioactive secondary volatile metabolites having antimicrobial properties. Similarly, the GCMS analysis of the crude extracts of Streptomyces sp. UTMC 1334,50 Streptomyces achromogenes TCH4,51 and Streptomyces sp. VITGV0152 belonging to actinomycetes identified a relatively high amount of aromatic volatile metabolites Pyrrolo[1,2 -a]pyrazine -1,4 -dione hexahydro – 3 -(2 -methylpropyl)- showing great antioxidant activity.
In context to GC-MS analysis, as reported earlier by investigators, the bioactive metabolites Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro- have antimicrobial as well as antioxidant activity.45-46 Also, some researchers found the volatile antifungal metabolite Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl), formed by Bacillus subtilis subsp. inaquosorum RLS76, played an significant role in controlling the growth of the Bt cotton phytopathogen Fov.47,49,51,52 But the two compounds 2,5-cyclohexadiene-1,4-dione, 2,5-Dihydroxy-3-methoxy-6-methyl- and 9,12-Octadecadienoic acid (Z, Z)-, phenylmethyl ester produced by Bacillus subtilis subsp. inaquosorum RLS76 were novel metabolites, and no such literature is available.
CAS plate assay for Siderophore
The biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 showed a transformation in color, shifting from blue to orange surrounding the inoculated colony with secretion and diffusion of siderophore compounds in CAS blue medium. (Photo Plate 2 and Table 4).
Table 4: Detection of siderophore production qualitatively by biocontrol agent using CAS agar plate method
| Sr. No. | Biocontrol agent | Colour of zone |
| 1 | Bacillus subtilis subsp. inaquosorum RLS76 | Orange |
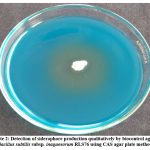 |
Plate 2: Detection of siderophore production qualitatively by biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 using CAS agar plate method. |
Siderophores, a small iron chelator molecule with low molecular weight, have a significant impact on inhibiting the growth of the fungal phytopathogen by engaging in iron competition.53 The six strains of Bacillus and two strains of Pseudomonas showed a color change from blue to an orange halo around the colonies on CAS agar plate after 5 days of incubation.54 The XN-04 strain of Streptomyces alfalfae, which is a type of actinomycetes, showed its ability to change the blue ferric CAS complex into a vibrant orange color. This change indicates that the strain is capable of producing siderophore.55 The Bacillus licheniformis YZCUO202005 strain 56 and Brevibacillus laterosporus 301/İK3-2 57 The blue color of the CAS agar plates was successfully altered, and the presence of a distinct orange to yellow halo on the medium indicated the production of siderophores.
The outcomes seen clearly demonstrated that our biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 effectively generated siderophore in CAS plates that were modified, resulting in a noticeable alteration of color in the CAS agar plate.
CAS Liquid Assay for Siderophore
The succinate broth inoculated with the biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 and incubated on a shaking incubator showed yellow colored growth (Photo Plate 3a). The color of the mixture consisting of 0.5 ml crude extract of Bacillus subtilis subsp. inaquosorum RLS76 and 0.5 ml of CAS solution changed from blue to orange (Photo Plate 3b). The percentage of siderophore units generated in the culture filtrate without cells was determined to be 86.06% after a duration of 60-72 hours (Table 5). During the subsequent incubation period, the quantity of siderophore units declined as a result of the degradation of the generated siderophore.
Table 5: Production of siderophore quantitatively by biocontrol agent by using liquid CAS assay
| Sr. No | Biocontrol agent | Siderophore units (%) | ||||
| 24 h | 36 h | 48 h | 60 h | 72 h | ||
| 1 | Bacillus subtilis subsp. inaquosorum RLS76 | 17.34 | 40.15 | 70.04 | 78.02 | 86.06 |
 |
Plate 3: (a) Growth of siderophore producing biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 in succinate broth (b) Detection of siderophore quantitatively by Bacillus subtilis subsp. inaquosorum RLS76 using liquid CAS assay. |
The rhizobacterial isolate Bacillus pumilus MK5 produced 51.36% siderophore units after 72 hours of incubation by liquid CAS assay.58 Novel strains of Bacillus cereus Wah1,59 Brevibacillus brevis GZDF3, 60 Bacillus subtilis subsp. inaquosorum RLS52,61 and Bacillus amyloliquefaciens subsp. amyloliquefaciens RLS19 62 also showed maximal siderophores production of 97.76, 29.67, 85.04, and 93.20% SU respectively. Also, the siderophore production of the Bacillus siamensis Gxun-6 strain 63 and Brevibacillus laterosporus 301/İK3-2 57 was detected by a liquid CAS assay. The tested strain was able to produce 56.43% and 50.00% siderophore units after 48 h of incubation respectively, showing that the bacteria had strong siderophore-producing ability.
In the present study, the Bacillus subtilis subsp. inaquosorum RLS76 strain produced 86.06% siderophore units after 72 h. These results were far better than the results documented by earlier research workers, except for Bacillus cereus Wah1 strain.59
Chitinase Enzyme Plate assay
The biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 showed a clear halo of distinct chitin hydrolysis surrounding the inoculated colony on colloidal chitin agar. The existence of the chitin hydrolysis enzyme is shown by the clear area surrounding the colony. The measurement of chitinase enzyme activity on the plate was recorded in millimeters (Table 6).
Table 6: Production of different enzymes by biocontrol agent.
| Sr. No. | Biocontrol agent | Hydrolytic enzyme activity | Total zone including colony (mm) | Diameter of colony (mm) | Diameter clear halo (mm) |
| 1 | Bacillus subtilis subsp. inaquosorum RLS76 | Chitinase enzyme activity on colloidal chitin agar | 29 | 13 | 16 |
| Protease enzyme activity by skim milk agar | 18 | 11 | 7 | ||
| Phosphate solubilization using NBRIP medium | 20 | 15 | 5 |
Chitin, an integral part of the fungal cell wall, is a plentiful natural polysaccharide consisting of a linear polymer of repeating β(1,4)-N-acetylglucosamine units. In nature, some microorganisms have the ability to use the substrate chitin by using enzymes called chitinases. The chitinase enzyme production ability of Bacillus spp. BT42 medium supplemented with colloidal chitin formed a noticeable clear zone surrounding the colony, indicating extracellular chitinase enzyme.64 Similarly, the Streptomyces alfalfae XN-04 strain on the colloidal chitin agar plate exhibited a clear halo of chitin hydrolysis of the inoculated bacterial culture.55 Also, Bacillus tequilensis strains B2 and B3 showed chitinase-producing ability on medium amended with colloidal chitin showing, a clear zone of clearance surrounding the inoculated colonies.65 This demonstrates the capability of these bacterial isolates to break down chitin, a crucial component of the fungal cell wall in Fusarium oxysporum.
In this study, the production of the chitinase enzyme by biocontrol agent Bacillus subtilis subsp. inaquosorum RLS76 was seen the visible zone of chitin hydrolysis. This confirms the significant role of the chitinase enzyme in controlling the phytopathogens Fusarium oxysporum f. sp. vasinfectum in cotton.
Protease Enzyme Plate assay
The biocontrol strain RLS76 of Bacillus subtilis subsp. inaquosorum exhibited the ability to produce extracellular protease enzyme, as evidenced by the clear halo around the inoculated colony on a skim-milk agar plate (Photo Plate 4 and Table 6).
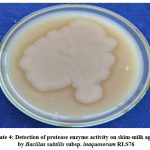 |
Plate 4: Detection of protease enzyme activity on skim-milk agar by Bacillus subtilis subsp. inaquosorum RLS76 |
Proteases are the most important enzymes produced by rhizobacterial microorganisms and used to control fungal phytopathogens. The Streptomyces alfalfae XN-04 grow well on medium amended with casein as a source of substrate, and a zone of clearance surrounding the colony indicated the protease enzyme production.55 Similarly, the strains of Bacillus tequilensis B2, B3, and B4 produced protease enzyme activity on a milk agar plate, which was indicated by the clear zone surrounding the inoculated cultures.65 Also, the PDA agar plate added with skimmed milk and spot inoculated with Bacillus licheniformis YZCUO202005 showed a visible clear halo zone of protein degradation surrounding the colony, indicating a strong protease enzyme activity.56
All these reports support the idea that the protease enzyme produced by Bacillus subtilis subsp. inaquosorum RLS76 played a significant role in inhibiting the mycelial growth of Fusarium wilt phytopathogen of Bt cotton.
Phosphate Solubilization Assay
The Bacillus subtilis subsp. inaquosorum RLS76 exhibited phosphate solubilization activity with the clear halo around the colonies (Photo Plate 5). The diameter of a clear halo surrounding the inoculated colony was measured in mm and found to be 5 mm (Table 6).
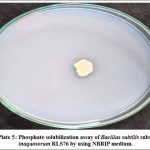 |
Photo Plate 5: Phosphate solubilization assay of Bacillus subtilis subsp. inaquosorum RLS76 by using NBRIP medium. |
Plants need phosphorous for their growth and development. The soil contains both organic and inorganic compounds of phosphate, but the majority of them are inactive and therefore not accessible to plants. Some rhizospheric microorganisms are able to solubilize organic and inorganic phosphate and make it available to plants.66 Similarly, the four strains of Sporolactobacillus laevolacticus exhibited a halo zone of tri-calcium phosphate solubilization on Pikovskaya’s agar plates, measuring over 5 mm in diameter after 9 days of incubation.67
Also, the Streptomyces alfalfae XN-04 exhibited robust growth on NBRIP medium supplemented with insoluble organic phosphate. This leads to the formation of a clear halo zone surrounding the colony, indicating the hydrolysis of the substrate and showcasing its capability to solubilize phosphate.55 Khatoon and Khan 68 showed that thirteen rhizospheric isolates solubilized phosphate on NBRIP agar medium supplemented with tri-calcium phosphate. Similarly, Bacillus licheniformis YZCUO202005 showed a visible halo zone of phosphate solubilization on NBRIP medium.56
All these reports gave positive evidence that Bacillus subtilis subsp. inaquosorum RLS76 possesses the capability to transform insoluble phosphate into soluble phosphate. This transformation is crucial in facilitating the growth promotion of Bt cotton crop.
Conclusion
The effectiveness of the rhizobacterial isolate Bacillus subtilis subsp. inaquosorum RLS76 as a biocontrol agent against Fusarium oxysporum f. sp. vasinfectum, a phytopathogen causing Fusarium wilt in Bt cotton, was proved in the present study. The findings clearly showed that the rhizobacterial isolate RLS76 effectively suppressed the radial growth of the phytopathogen Fusarium oxysporum f. sp. vasinfectum, with an impressive inhibition rate of 85.39%. The rhizobacterial isolate displayed various direct and indirect mechanisms for biocontrol and plant growth promotion, including diffusible nonvolatile antifungal organic compounds and volatile organic compounds. The GC-MS analysis of the crude extract of the isolate identified four compounds, namely Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-; Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl); 2,5-cyclohexadiene-1,4-dione, 2,5-Dihydroxy-3-methoxy-6-methyl- and 9,12-Octadecadienoic acid (Z,Z)-,phenylmethyl ester. Out of four compounds, three show antimicrobial activity except 2,5-Dihydroxy-3-methoxy-6-methyl. Also, the rhizobacterial isolate was able to produce siderophore and showed strong chitinase, protease and phosphate solubilization activity. Based on the findings mentioned earlier, it has been concluded that our rhizobacterial isolate, Bacillus subtilis subsp. inaquosorum RLS76 demonstrates remarkable potential as a biocontrol agent and plant growth-promoting candidate in the effective management of Fusarium wilt disease in Bt-Cotton.
Acknowledgement
We acknowledge the Principal, Sant Tukaram College of Arts and Science, Basmat Road, Parbhani-431401 (M.S.) India for supplying us the good laboratory facilities during this research work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare no conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Approval Statement
‘Not applicable’ (No need for ethical approval).
Authors’ Contribution
Laxman S. Raut
Formulated and executed the analysis, conducted all practical tasks, and drafted the manuscript.
Sanjay M. Dalvi
Assessed and revised the manuscript.
Ravindra R. Rakh
Devised and planned the analysis, and formatted the manuscript according to journal guidelines.
References
- Cianchetta A. N., Davis R. M. Fusarium wilt of cotton: Management strategies. Crop Protection. 2015;73:40-44. DOI: 1016/j.cropro.2015.01.014
CrossRef - Patel B. K., Sandipan P. B., Patel R. K., Chawada S. K. Screening of different fungicides and biocontrol agents against Fusarium oxysporum sp. vasinfectum (FOV) under pot condition. IJCS.2021;9(1):1005-1007. DOI: 10.22271/chemi.2021.v9.i1n.11358
CrossRef - Asif R., Siddique M. H., Zakki S. A., Rasool M. H., Waseem M., Hayat S., Muzammil S. Saccharothrix Algeriensis NRRL B-24137 potentiates chemical fungicide carbendazim in treating Fusarium Oxysporum sp. Vasinfectum-induced cotton wilt disease. Dose-Response. 2020; 18(3):1-9. 1559325820960346. DOI: 10.1177/1559325820960346
CrossRef - Patel B. K., Sandipan P. B., Patel R. K., Chawada S. K. Screening of different non systemic and systemic fungicides for the wilt disease of cotton under in vitro condition of south Gujarat. Int J Curr Microbiol App Sci. 2020;9(12): 820-825.DOI: 20546/ijcmas.2020.912.098
CrossRef - Rajput A. Q., Arain M. H., Pathan M. A., Jiskani M. M., Lodhi A. M. Efficacy of different fungicides against Fusarium wilt of cotton caused by Fusarium oxysporum sp. vasinfectum.Pakistan Journal of Botany. 2006;38(3):875-880.
- Chowdhury S. K., Majumdar S., Mandal V. Application of Bacillus sp. LBF-01 in Capsicum annuum plant reduces the fungicide use against Fusarium oxysporum. Biocatalysis and Agricultural Biotechnology. 2020;27:101714. DOI: 1016/j.bcab.2020.101714
CrossRef - He P., Li S., Xu S., Fan H., Wang Y., Zhou W., Fu G., Han G., Wang Y. Y. Zheng, S. J. Monitoring tritrophic biocontrol interactions between Bacillus spp., Fusarium oxysporum sp. cubense, tropical race 4, and banana plants in vivo based on fluorescent transformation system. Frontiers in Microbiology. 2021;12:754918. DOI: 10.3389/fmicb.2021.754918
CrossRef - Banerjee S., Singh S., Pandey S., Bhandari M. S., Pandey A., Giri K. Biocontrol potential of Pseudomonas azotoformans, Serratia marcescens and Trichoderma virens against Fusarium wilt of Dalbergia sissoo. Forest Pathology. 2020;50(2): e12581. DOI: 1111/efp.12581
CrossRef - Farfour S. A., El-Ghanam A. A., Hamouda R. A., Darwish D. B., Al-Saman M. A. Pseudomonas spp., promising biocontrol agents against pre and post-harvest Fusarium wilt disease in Cucumis melo. fruits. Biosci Res. 2021;18(4):2553-2561.
- Mehmood N., Saeed M., Zafarullah S., Hyder S., Rizvi Z. F., Gondal A. S., Jamil N., Iqbal R., Ali B., Ercisli S., Kupe M. Multifaceted impacts of plant-beneficial Pseudomonas spp. in managing various plant diseases and crop yield improvement. ACS omega. 2023;8(25):22296-22315. DOI: 1021/acsomega.3c00870
CrossRef - Li Z., Ma J., Li J., Chen Y., Xie Z., Tian Y., Su X., Tian T., Shen T. A biocontrol strain of Serratia plymuthica MM promotes growth and controls Fusarium Wilt in watermelon. Agronomy. 2023;13(9):2437. DOI: 3390/agronomy13092437
CrossRef - Hilaire K. T., Antoine B. B. B., Faustin S. D., Souleymane C., Laurent K. K., Tchoa K., Daouda K. Biopotential of some Trichoderma spp. against growth of Fusarium oxysporum sp. vasinfectum causal agent of cotton wilt. British Microbiology Research Journal. 2015;10(5): 1-11. DOI:10.9734/BMRJ/2015/20518
CrossRef - Damodaran T., Rajan S., Muthukumar M., Gopal R., Yadav K., Kumar S., Ahmad I., Kumari N., Mishra V. K., Jha S. K. Biological management of banana Fusarium wilt caused by Fusarium oxysporum sp. cubense tropical race 4 using antagonistic fungal isolate CSR-T-3 (Trichoderma reesei). Frontiers in Microbiology. 2020;11:595845. DOI: 10.3389/fmicb.2020.595845
CrossRef - Kutama A. S., Aliyu U., Sultan Z., Ali B. A., Musa H. M. In vitro inhibitory potential of Trichoderma species on Fusarium oxysporum sp vasinfectum the causal organism of vascular wilt of cotton (Gossypium hirsutum l.) in the Nigerian Sudan Savanna. UMYU Scientifica. 2022;1(1):122-126. DOI: 10.56919/usci.1122.016
CrossRef - Brizuela A. M., Gálvez L., Arroyo J. M., Sánchez S., Palmero D. Evaluation of Trichoderma spp. on Fusarium oxysporum sp. asparagi and Fusarium wilt Control in Asparagus Crop. Plants. 2023;12(15):2846. DOI: 10.3390/plants12152846
CrossRef - Aydi-Ben-Abdallah R., Jabnoun-Khiareddine H., Daami-Remadi M. Fusarium wilt biocontrol and tomato growth stimulation, using endophytic bacteria naturally associated with Solanum sodomaeum and bonariense plants. Egyptian Journal of Biological Pest Control. 2020;30:1-13. DOI: 10.1186/s41938-020-00313-1
CrossRef - Maulidia V., Sriwati R., Soesanto L., Syamsuddin Hamaguchi T., Hasegawa K. Endophytic bacteria isolated from higher plant in Aceh, Indonesia, and their chemical compounds activity against Fusarium oxysporum sp. lycopersici. Egyptian Journal of Biological Pest Control. 2021;31:31. DOI: 10.1186/s41938-021-00379-5
CrossRef - Taribuka J., Wibowo A., Widyastuti S. M., Sumardiyono C. Potency of six isolates of biocontrol agents endophytic Trichoderma against fusarium wilt on banana. Journal of Degraded and Mining Lands Management. 2017;4(2):723-731. DOI: 15243/jdmlm.2017.042.723
CrossRef - Rajani P., Rajasekaran C., Vasanthakumari M. M., Olsson S. B., Ravikanth G., Shaanker R. U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiological Research. 2021;242: 126595. DOI: 1016/j.micres.2020.126595
CrossRef - Dennis C., and Webster J. Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Transactions of the British Mycological Society. 1971; 57(1):41-48. DOI: 1016/S0007-1536(71)80078-5
CrossRef - Skidmore A. M., Dickinson C. H. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Transactions of the British Mycological Society. 1976;66(1):57-64. DOI: 1016/S0007-1536(76)80092-7
CrossRef - Rawlings D. E. Restriction enzyme analysis of 16S rRNA genes for the rapid identification of Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans strains in leaching environments. Biohydrometallurgical processing. 1995;2:9-17.
- Muyzer G., De Waal E. C., Uitterlinden A. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Applied and Environmental Microbiology.1993;59(3):695-700. DOI: 1128/aem.59.3.695-700.1993
CrossRef - Schlumbaum A., Mauch F., Vogeli U. and Boller T. Plant chitinases are potent inhibitors of fungal growth. Nature. 1986;324:365-367. DOI: 1038/324365a0
CrossRef - Whipps J. M. Effect of media on growth and interactions between a range of soil‐borne glasshouse pathogens and antagonistic fungi. New Phytologist. 1987;107(1):127-142. DOI: 1111/j.1469-8137.1987.tb04887.x
CrossRef - Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Analytical biochemistry. 1987;160(1):47-56. DOI: 1016/0003-2697(87)90612-9
CrossRef - Meyer J. A., Abdallah M. A., The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. Microbiology. 1978;107(2):319-328. DOI: 1099/00221287-107-2-319
CrossRef - Payne S. M. [25] Detection, isolation, and characterization of siderophores. In Methods in enzymology. Academic Press. 1994; 235:329-344. DOI: 1016/0076-6879(94)35151-1
CrossRef - Hsu S. C., Lockwood J. L. Powdered chitin agar as a selective medium for enumeration of actinomycetes in water and soil. Applied Microbiology. 1975;29(3): 422-426. DOI: 1128/am.29.3.422-426.1975
CrossRef - Abdelmoteleb A., Troncoso-Rojas R., Gonzalez-Soto T., González-Mendoza D. Antifungical activity of autochthonous Bacillus subtilis isolated from Prosopis juliflora against phytopathogenic fungi. Mycobiology, 2017; 45(4): 385-391. DOI: 5941/MYCO.2017.45.4.385
CrossRef - Nautiyal C. S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters. 1999;170(1):265-270. DOI: 1111/j.1574-6968.1999.tb13383.x
CrossRef - Gajbhiye A., Rai A. R., Meshram S. U., Dongre A. B. Isolation, evaluation and characterization of Bacillus subtilis from cotton rhizospheric soil with biocontrol activity against Fusarium oxysporum. World Journal of Microbiology and Biotechnology. 2010;26:1187-1194. DOI: 1007/s11274-009-0287-9
CrossRef - Zain M., Yasmin S., Hafeez F. Y. Isolation and characterization of plant growth promoting antagonistic bacteria from cotton and sugarcane plants for suppression of phytopathogenic FusariumIranian journal of biotechnology. 2019;17(2):e1974. DOI: 10.21859/ijb.1974
CrossRef - Murty L. D., Shim W. B. Streptomyces and Bacillus species utilize volatile organic compounds to impact Fusarium oxysporum sp. vasinfectum race 4 (Fov4) virulence and suppress Fusarium wilt in Pima cotton. BioRxiv. 2021;1-34. DOI: 10.1101/2021.10.27.466178
CrossRef - Keswani C., Singh H. B., García-Estrada C., Caradus J., He Y. W., Mezaache-Aichour S., Glare T. R., Borriss R., Sansinenea E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Applied microbiology and biotechnology. 2020;104:1013-1034. DOI: 1007/s00253-019-10300-8
CrossRef - Mardanova A. M., Hadieva G. F., Lutfullin, M. T., Khilyas I. V. E., Minnullina L. F., Gilyazeva A. G., Bogomolnaya L. M., Sharipova M. R. Bacillus subtilis strains with antifungal activity against the phytopathogenic fungi. Agricultural Sciences. 2016;8(1):1-20. DOI: 4236/as.2017.81001
CrossRef - Ben Ayed H., Hmidet N., Béchet M., Jacques P., Nasri M. Identification and natural functions of cyclic lipopeptides from Bacillus amyloliquefaciensEngineering in Life Sciences. 2017;17(5):536-544. doi: 10.1002/elsc.201600050
CrossRef - Al-Mutar D. M. K., Alzawar N. S. A., Noman M., Azizullah Li D., Song F. Suppression of fusarium wilt in watermelon by Bacillus amyloliquefaciens DHA55 through extracellular production of antifungal Lipopeptides. Journal of Fungi. 2023;9(3):336.01-22. DOI: 3390/jof9030336
CrossRef - Montalvão S. C., Castro M. T. D., Blum L. E., Monnerat R. G. Biocontrol of Fusarium oxysporum sp. vasinfectum With Bacillus spp. Strains. Journal of Agricultural Science.2021;13(9):1-16. DOI: 10.5539/jas.v13n9p1
CrossRef - Rafanomezantsoa P., Gharbi S., Karkachi N., Kihal M. Antifungal activity of Bacillus spp. against Fusarium oxysporum sp. lycopersici and Ascochyta sp. Journal of Plant Protection Research. 2022;62(3):247–257. DOI: 10.24425/jppr.2022.142131
CrossRef - Russi A., Almanca M. A. K., Schwambach J. Bacillus subtilis strain F62 against Fusarium oxysporum and promoting plant growth in the grapevine rootstock SO4. Anais da Academia Brasileira de Ciências. 2022;94(3):01-13. DOI: 1590/0001-3765202220210860
CrossRef - Voisard C., Bull C. T., Keel C., Laville J., Maurhofer M., Schnider U., Défago G., Haas, D. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches. Molecular ecology of rhizosphere microorganisms. 1994;2:67-89.
CrossRef - Howell C. R., Beier R. C., Stipanovic R. D. Production of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology. 1988;78(8):1075-1078. DOI: 1094/Phyto-78-1075
CrossRef - Dowling D. N., O’Gara F. Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends in Biotechnology. 1994;12(4):133-141. DOI:1016/0167-7799(94)90091-4
CrossRef - Ser H. L., Palanisamy U. D., Yin W. F., Abd Malek S. N., Chan K. G., Goh B. H., Lee L. H. Presence of antioxidative agent, Pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro-in newly isolated Streptomyces mangrovisoli sp. nov. Frontiers in Microbiology. 2015;6:854. 3389/fmicb.2015.00854
CrossRef - Kiran G. S., Priyadharsini S., Sajayan A., Ravindran A., Selvin J. An antibiotic agent pyrrolo [1, 2-a] pyrazine-1, 4-dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC advances. 2018;8(32):17837-17846. DOI: 1039/C8RA00820E
CrossRef - Malash M. A., El-Nagga M. M., Abou El Hassayeb H. E., Ibrahim M. S. Production of Antimicrobial Pyrrol-Derevatives Acting Against Some Fish Pathogens from Marine mmm. Global Veterinaria. 2016;17:495-504. DOI: 5829/idosi.gv.2016.495.504
- Raut L.S., Hamde V.S. In vitro antagonism of resident rhizobacteria, Bacillus amyloliquefaciens amyloliquefaciens subsp. amyloliquefaciens against the bacterial blight pathogen of Bt cotton. International Journal of Pharm Bio Sciences. 2018;8(61):1-6. URL: 5b9246bedffc9.pdf
- Hallaj-Nezhadi S., Hamdipour R., Shahrvirani M., Zare tin R., Chapeland-Leclerc F., Ruprich-Robert G., Esnaashari S., Dilmaghani A Antimicrobial activity of Bacillus sp. isolated strains of wild honey. BMC Complementary Medicine and Therapies. 2022;22(1):78. DOI: 1186/s12906-022-03551-y
CrossRef - Almasi F., Mohammadipanah F., Adhami H. R., Hamedi J. Introduction of marine‐derived Streptomyces sp. UTMC 1334 as a source of pyrrole derivatives with anti‐acetylcholinesterase activity. Journal of applied microbiology. 2018;125(5):1370-1382. DOI: 1111/jam.14043
CrossRef - Tangjitjaroenkun J., Pluempanupat W., Tangchitcharoenkhul R., Yahayo W., Supabphol R. Antibacterial, antioxidant, cytotoxic effects and GC-MS analysis of mangrove-derived Streptomyces achromogenes TCH4 extract. Archives of Biological Sciences. 2021;73(2):223-235. DOI:2298/ABS210320017T
CrossRef - Veilumuthu P., Godwin Christopher J. Antimicrobial compounds produced by Streptomyces sp. VITGV01 against selected human pathogens. Research Journal of Biotechnology. 2022;17(12):16-28. DOI: 25303/1712rjbt16028
CrossRef - Whipps J. M. Microbial interactions and biocontrol in the rhizosphere. Journal of experimental Botany. 2001;52(1):487-511. DOI: 1093/jexbot/52.suppl_1.487
CrossRef - Ghazy N., El-Nahrawy S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Archives of Microbiology. 202;203:1195-1209.DOI: 1007/s00203-020-02113-5
CrossRef - Chen J., Hu L., Chen N., Jia R., Ma Q., Wang Y. The biocontrol and plant growth-promoting properties of Streptomyces alfalfae XN-04 revealed by functional and genomic analysis. Frontiers in Microbiology. 2021;12:745766. DOI: 3389/fmicb.2021.745766
CrossRef - Medison R. G., Jiang J., Medison M. B., Tan L. T., Kayange C. D., Sun Z., Zhou Y. Evaluating the potential of Bacillus licheniformis YZCUO202005 isolated from lichens in maize growth promotion and biocontrol. Heliyon. 2023;9(10): DOI: 10.1016/j.heliyon.2023.e20204
CrossRef - Özdemir F. İ., Aydin B., Tülek A. Investigation of the siderophore production and associated heavy metal accumulation potential of Brevibacillus laterosporus 301/İK3-2. Hacettepe Journal of Biology and Chemistry. 2023;51(3):317-325. DOI: 15671/hjbc.1256836
CrossRef - Kaushal M., Kaushal R. Acetylene reductase activity and molecular characterization of plant growth promoting rhizobacteria to know efficacy in integrated nutrient management system. Indian Journal of Biotechnology. 2015;14(2):221-227. http://oar.icrisat.org/id/eprint/9122
- Maleki M., Norouzpour S., Rezvannejad E., Shakeri S. Novel strains of Bacillus cereus Wah1 and Enterobacter cloacae Wkh with high potential for production of siderophores. Biological Journal of Microorganism. 2017;6(24):1-11.
- Sheng M., Jia H., Zhang G., Zeng L., Zhang T., Long Y., Lan J., Hu Q., Zeng Z., Wang B., Liu, H. Siderophore production by rhizosphere biological control bacteria Brevibacillus brevis GZDF3 of Pinellia ternata and its antifungal effects on Candida albicans. Journal of Microbiology and Biotechnology. 2020;30(5):689-699. DOI: 4014/jmb.1910.10066
CrossRef - Raut L. S., Rakh R. R., Hamde V. S. In vitro microbiological control of Alternaria macrospora, a leaf spot pathogen of Bt cotton with Bacillus subtilis subsp. inaquosorum Res J Agric Sci. 2020;11:424-429. http://rjas.org/ViewIssue?IssueId=67
- Raut L. S., Rakh R. R., Hamde V. S. In vitro biocontrol scenarios of Bacillus amyloliquefaciens subsp. amyloliquefaciens strain RLS19 in response to Alternaria macrospora, an Alternaria leaf spot phytopathogen of Bt cotton. Journal of Applied Biology and Biotechnology. 2021;9(1):75-82. DOI: 7324/JABB.2021.9110
CrossRef - Shen N., Li S., Li S., Zhang H., Jiang M. The siderophore-producing bacterium, Bacillus siamensis Gxun-6, has an antifungal activity against Fusarium oxysporum and promotes the growth of banana. Egyptian Journal of Biological Pest Control. 2022;32(1):34. DOI: 1186/s41938-022-00533-7
CrossRef - Kejela T., Thakkar V. R., Thakor P. Bacillus species (BT42) isolated from Coffea arabica L. rhizosphere antagonizes Colletotrichum gloeosporioides and Fusarium oxysporum and also exhibits multiple plant growth promoting activity. BMC Microbiology. 2016;16:1-13. DOI: 1186/s12866-016-0897-y
CrossRef - Baard V., Bakare O. O., Daniel A. I., Nkomo M., Gokul A., Keyster M., Klein A. Biocontrol potential of Bacillus subtilis and Bacillus tequilensis against four fusarium species. 2023;12(2):254. DOI: 10.3390/pathogens12020254
CrossRef - Tian J., Ge F., Zhang D., Deng S., Liu X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology. 2021;10(2):158. DOI: 3390/biology10020158
CrossRef - Rakh R. R., Raut L. S., Dalvi S. M. In vitro vetting for phosphate solubilizing bacteria (PSB) from healthy plants rhizospheric niches. Res Jr of Agril Sci. 2021;12(1):331–337.
- Khatoon N., Khan M. Evaluation of Bacillus subtilis MRB4, as plant growth promoter and potential phosphate solubilizer under abiotic stress. Journal of Applied Biology and Biotechnology. 2020;8(5):27-35. DOI: 7324/JABB.2020.80504
CrossRef - Sivakrishnan S., Kottaimuthu A. Hepatoprotective activity of ethanolic extract of aerial parts of Albizia procera Roxb (Benth.) against paracetamol induced liver toxicity on Wistar rats. International Journal of Pharmacy and Pharmaceutical Science. 2014;6(1): 233-238.

