Introduction
Coffee is one of the world’s most important agricultural commodities 1. It is planted in more than 10 million hectares over 50 countries 2. Approximately, 125 million people in Latin America, Asia and Africa are deriving their livelihood directly or indirectly from its products 3. About 20% to 40% of the world’s crop production is lost annually to pests and diseases. Commercial coffee production levels rely on two species Coffea Arabica and Coffea Canephora. Higher quality coffee is associated with C. Arabica and this variety represents 73% of world production in Latin America 4. Kenya, which is the origin of Robusta coffee, produces the best Robusta in the world 5. Coffee farming in Kenya is mainly done by small-scale farmers organized into co-operative societies who account for 60% while 40% is done by large scale farmers at plantation or estate levels 6. In Kenya, coffee contributed an average of 60% foreign exchange earnings until the year 2002 when its contribution fell to a mere 25%. This rapid fall brought about social and economic imbalances in more than 3,000,000 small scale holders affecting their daily livelihoods 7. The crisis facing the coffee industry has been characterized by collapsing prices, deteriorating coffee quality, diseases and above all the growing inequality in the coffee value chain (United Nations conference on trade and development) 5.
Kirinyaga is one of the largest coffee growing Counties in Kenya. It has 44, 969 acres of land under coffee with a population of 67,578 coffee farmers 8. The coffee industry in Kirinyaga has created employment for many people since coffee is produced by small- scale holders. The coffee estates, cooperatives and factories in Kirinyaga have employed several people. The coffee estates, cooperatives and factories in Kirinyaga include; Kibirigwi Farmers’ Cooperative Society, Rung’eto Farmers’ Co-operative Society, Karimikui and Kiangoi Factories 9.
Coffee production in Kirinyaga County has remained low since 2002. The farmers are incurring heavy losses from the farm inputs including fertilizers and pesticides required in coffee production 10. The farmers of late have been complaining of huge losses following an outbreak of coffee diseases 10. The crop has been affected by Coffee Berry Disease (CBD), a fungal infection associated with cold weather, and bacterial blight disease. CBD attacks young green berries, and if uncontrolled can lead to loss of an entire crop 11. Farmers depend heavily on fungicides and pesticides for controlling the badly ravaging diseases and pest infestation of coffee. Farms do up to eight rounds of copper sprays in the management of coffee diseases. Pesticides benefit the crops; however, they also impose a serious negative impact on the environment. Excessive use of pesticides may lead to the destruction of biodiversity. The survival of many birds, aquatic organisms, microorganisms and animals is under the threat of harmful pesticides. These harmful effects are a concern for sustainability of the environment and global stability 12.
Coffee processing plants often discharge waste into rivers creating pollution and contamination problems that can cause eutrophication of the water systems and kill aquatic plants and animals 13. Human societies rely on the vast diversity of benefits provided by nature, such as food, fibers, construction materials, clean water, clean air and climate regulation. All the elements required for these ecosystem services depend on soil, and soil biodiversity is the driving force behind their regulation 14. Fungi are some of the most diverse microorganisms that inhabit different environmental sources such as soil, plant parts (leaves, roots, fruits) water and food. Fungi are the major decomposers of dead organic matter and contribute significantly to the recycling of nutrients in natural and modified ecosystems. The growth and distribution of fungi are affected by different environmental factors such as temperature, pH, moisture, degree of aeration, amount and type of nutrients, other different soil properties and human activities 15. The real number of fungi is still unknown. Only 5-13% of the fungal species evaluated worldwide have been characterized 16. Understanding the diversity of microbial communities is an ecological priority 17. Thus, their isolation and identification from different environmental sources is still very essential for viewing and recognizing of more species and editing scientific classification. In addition, their identification will be important in evaluating their effects in nature and supplying strains for ecological remediation, biological control and industrial aspects 18.
Most farmers in Kirinyaga County have traditional varieties of coffee that succumb to coffee diseases. Their management of the diseases over the years has been the use of chemicals which in turn affect the distribution of growth promoting organisms in the soil This study is therefore very important to determine the status of soil and plants (berries) structures richness in diversity and distribution of different fungi in coffee farms Kirinyaga County in Kenya. This will be an avenue to know the richness of soil microorganisms for future sustainability of coffee cultivation. It will provide information on other sustainable disease management and pest management programs like biological control, because the findings of this study can contribute to biological control studies.
Material and Methods
Sampling Location
This study was carried out in Kirinyaga County, Kenya in 4 Sub counties (figure 1). The County is located on the South-Eastern slopes of Mt Kenya in the Central part of Kenya. It lies between latitude 0.6591°S and longitude 37.3827°E. The mean temperature of the coffee growing zone in the county is 20°C with a range of 12°C to 26°C 19. The mean monthly temperature in the area around the lower zones is approximately 22.2°C with a minimum of 21.8°C in January and 24°C in March 20. A total of 100 farms were selected. The sub-counties involved were Kirinyaga East 25 farms, Kirinyaga West 44 farms, Kirinyaga Central 28 farms and 3 farms from Mwea East.
The farms were randomly selected depending on their availability at an interval of 1 km apart along a transect that cuts across the three upper midland zones: – Upper Midland zone 1{UM 1}, Upper Midland zone 2 {UM 2}) and Upper Midland zone 3 {UM 3}), altitude (Table 1) and Sub-counties as indicated in table 2.
Table 1: Description of Coffee Agro-Ecological Zones in Kenya. Jaetzold et al., (2006).
| Agro-Ecological Zones | Altitude (Meters above sea level) | Mean Temperature (°C) | Mean annual rainfall (mm) | Description |
| Upper Midland zone 1 | 1520-1820 | 18.4 | 1550 | Coffee- Tea zone |
| Upper Midland zone 2 | 1400-1580 | 19.5 | 1360 | Main Coffee zone |
| Upper Midland zone 3 | 1310-1400 | 20.3 | 1175 | Marginal coffee |
Table 2: Distribution of Sampled Farms Across the Agro Ecological Zones and Sub-Counties.
| Agro-Ecological Zones | Sub-county | Number of farms |
| Upper Midland 1 | Kirinyaga East | 22 |
| 18 | ||
| 20 | ||
| Upper Midland 2 | Kirinyaga East | 3 |
| Kirinyaga Central | 8 | |
| Kirinyaga West | 20 | |
| Upper Midland 3 | 2 | |
| 4 | ||
| 3 |
 |
Figure 1: Geographical Locality Map of the Sampling Sites showing the Five Sub-Counties of Kirinyaga (Stars) Map Modified from 20. |
Sample Collection
Plant residues were removed from the soil surface, and a soil auger used to scoop soil from the plough layer (0-15 cm in depth). Soil sampling was done using a Z shape method from 7 different points of the farm as described by Gaddeyya 15. The sample was then thoroughly mixed in a labeled sample bag and transported to the laboratory for isolation of microbes.
Sampling of Coffee Berries
Berries at an expanding stage were handpicked from coffee plants following a Z shape pattern around the farm. A minimum of 10 berries was collected from each tree. The berries were then put in a plastic bag with labels indicating the name of the farmer, farm number and date of sampling. These were taken to plant pathology laboratory in Coffee Research Institute in Ruiru in different batches for isolation of the surface microbes.
Media Preparation
Fifteen grams of commercially prepared dehydrated potato dextrose agar (PDA) was weighed into a flat-bottomed flask. Four hundred (400) ml of distilled water was added into the flask and swirled to dissolve. The mixture was then autoclaved at 121°C for 15 minutes. The mixture was then allowed to cool to room temperature. It was then dispensed to half fill 9 cm- Petri dishes. This was repeatedly done to cater to all the Petri dishes required for all the samples. This was done in aseptic condition to avoid contamination in repetitive measures for culturing all the samples.
Isolation of Fungi from Soil
Soil fungi were isolated by serial dilution method 21. To obtain stock solution, 1g of the soil was added to 10 ml of distilled water in sterilized McCartney tubes and gently shaken for better distribution of soil particles. Serial dilutions were prepared from the stock suspension to obtain 10-1, 10-2 and 10-3 but only 10-3 was plated on the culture media. One (1) ml of the medium suspension was drawn into a pipette from the weakest dilution (10-3), and dispensed into each of the three replicas Petri dishes (Labelled 10-3) with the solidified PDA media. This was spread over the entire surface using an L- stirring rod. The whole procedure was then repeated for all the soil samples and replicated three times. The Petri dishes were then sealed with Para film. The inoculated plates were incubated at room temperature for ten days. The number of colonies was recorded per petri dish for each dilution. The representative colonies were then sub-cultured on solidified PDA media by scooping the mycelia from each colony and placing it into a separate Petri dish which was incubated for ten days for further identification. The characteristics of colonies obtained were also recorded for identification.
Fungal Isolation from Coffee Berries
The berries were washed each berry on its own in a conical flask using a drop of Teepol® detergent (Shell Chemicals Industrial Limited) and rinsed twice with double-distilled water. The berries were placed on moist wadding paper inside plastic lunch boxes to promote sporulation and incubated at room temperature. Berries with the best lesion were selected from the incubated berries for isolation of microbes. Isolation was carried out by excising the whole lesion and suspending it in 10 mls of sterile distilled water in a labeled McCartney tube. Serial dilution was made by drawing 1ml from the stock spore suspension medium and dispensed into 9ml distilled water, plated onto PDA in three replicates per dilution and incubated at room temperature for 10 days. The colonies were then sub-cultured and incubated for ten days for further identification as described by Watanable 22.
Identification of the Fungi
The identification of the fungi was based on their morphology on culture. The microscopic and morphological characteristics used were, presence or absence of aerial mycelium, the color, wrinkles/furrows and pigmentation 15.
Results
Morphological Characteristics of Isolated Fungi
The characteristics of the isolated fungi varied in texture, appearance, colour and margin as tabulated below in Table 3.
Table 3: Colony Characteristics of Fungi Isolated from Soils and Coffee Berries.
| Code | Fungi | Colony colour |
Texture | Appearance | Margin |
| A | Aspergillus | Green | Rough | Ovoid | Irregular |
| B | Trichoderma | Green | Rough | Ellipsoidal | Granular |
| C | Penicillium | Grey | Smooth | Brushlike | Rough walled |
| D | Epicoccum | Brown | Cottony | Spherical | Irregular |
| E | Cladosporium | Grey | Powdery | Geniculate | Feathery |
| F | Fusarium | Light purple | Fluffy | Elongated | Smooth |
| G | Alternaria | Greyish | Smooth | Ovoid | Irregular |
| H | Phoma | Grey | Rough | Ovoid | Irregular |
| I | Rhizopus | light brown | Smooth | Cylindrical | Irregular |
| J | Colletotrichum kahawae | Grey | Smooth | Cylindrical | Regular |
Plates A and B indicated different fades of green colour of Aspergillus and Trichoderma. Plates C, E, G, H and J representing Penicillium, Cladosporium, Alternaria, Phoma and Colletotrichum kahawae respectively were grey colour. The texture of the fungal colonies varied from rough, smooth, cottony, powdery to fluffy Figure 2 at a magnification of X10.
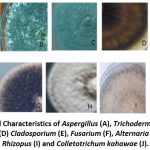 |
Figure 2: Cultural Characteristics of Aspergillus (A), Trichoderma (B), Penicillium (C), Epicoccum (D) Cladosporium (E), Fusarium (F), Alternaria (G), Phoma (H), Rhizopus (I) and Colletotrichum kahawae (J). |
Microscopic Features of the Isolated Fungi
The conidial arrangement of the fungi isolated from the soil and coffee berry samples as observed under the microscope are shown in Figure 3 at a magnification of X10.
 |
Figure 3: Microscopy Photography showing Conidia Arrangement of Aspergillus (A), Trichoderma (B), Penicillium (C), Epicoccum (D), Cladosporium (E), Fusarium (F), Alternaria (G), Phoma (H), Rhizopus (I) and Colletotrichum kahawae (J). |
The features seen varied in the forms of the conidiophores for the different fungi. Aspergillus as seen in plate A, presented long smooth brown conidiophores originating from basal foot cell. Trichoderma (Plate B) exhibited upright conidiophores, one single cell the conidia was ovoid and elongated hyphae. In Plate C, Penicillium conidiophores arising from mycelium and branching near the apex to forming a brush like structure were observed. Epicoccum, in Plate D had brown septate hyphae, short conidiophores (branching repeatedly originating from the hyphae. Plate E representing Cladosporium had erect aseptate and non-spherical conidiophores. Fusarium had straight to slightly curved macro conidia, oval to kidney shaped abundant microconidia (Plate F). Plate G shows Alternaria which had hyphae and brown septate conidiophores with simple conidia producing germ tubes. Phoma was seen with septate hyphae. Conidiophores having different lengths and are more thickened than mycelial hyphe (Plate H). Plate I showed Rhizopus which were non-septate with broad hyphae. The sporangiophores were unbranched and formed clusters. Colletotrichum kahawae represented in Plate J showed septate conidioshpores.
Fungal Diversity on Coffee Berries and Soils
A Total of 10 fungi were isolated from the samples collected. Epicoccum was the least prevalent fungi on average in all the sub-counties as seen in the graph plotted below figure 4 on microorganism against their percentage presence.
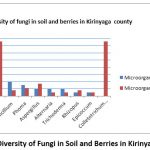 |
Figure 4: Diversity of Fungi in Soil and Berries in Kirinyaga County. |
Colletotrichum kahawae (76%), Cladosporium (76%) and Fusarium (73%) were the most common fungi isolated from the coffee berries while Fusarium was the most common in the soil samples (50%). Colletotrichum kahawae was not isolated from the soil samples under the conditions of this study. Epicoccum was the least prevalent fungi on average in all the sub-counties as seen in the graph plotted below on microorganism against their percentage presence.
Fungi Isolate from Soil
The fungal isolates from the respective counties indicated different proportions of prevalence.
Penicillium and Cladosporium at 35 % and 23 % respectively were the most prevalent in Kirinyaga Central whereas Alternaria was least prevalent at 3%. Aspergillus and Cladosporium were the most abundant in Kirinyaga East and Trichoderma being the least prevalent. Penicilliun, Aspergillus and Cladosporium were the most prevalent in Kirinyaga west and Alternaria least prevalent. Aspergillus was the most prevalent fungi in Mwea East. Trichoderma and Alternaria were the least prevalent.
Fungi Isolated from Berries
The results indicated that, the fungal isolates from berries that were abundant in all the subcounties were Colletotrichum kahawae, Fusarium and Cladosporium, with Eppicocum and Rhizopus being the least. Colletotrichum kahawae was most abundant in Kirinyaga Central at 42% and the least being Alternaria.
Fusarium and cladosporium was the most prevalent fungi in Kirinyaga East. The prevalence of some fungi was low i.e Penicillium 2%, Epicoccum 2%, Alternaria 2%, Phoma 3% and Rhizopus 1%. Colletotrichum was the most prevalent fungal isolate from Kirinyaga west with Epicoccum and and Phoma being the least prevalent. Colletotrichum kahawae and Cladosporium dominated in same proportions in Mwea east.
Discussion
Diversity of Microorganisms
Ten fungi isolated from Kirinyaga were identified up to genus level using morphological characteristics and observation under the compound microscope. The isolated fungi belong to four classes as follows; Eurotinimycetes (Aspergillus and Penicillium), Sordariomycetes (Fusarium, Trichoderma and C. kahawae) and Dothideomycetes (Alternaria, Epicoccum, Cladosporium and Phoma), Mucoromycotina (Rhizopus). Eight of the isolated fungi were isolated from both soil and berries whereas only two isolates i.e. Colletotrichum kahawae and Epicoccum were isolated from the coffee berries only.
Diversity in Soils and Berries and Agro-Ecological Zones
Out of the 10 genera distinguished, Colletotrichum and Fusarium presented the highest diversity richness in berries. Cladosporium, Penicillium and Aspergillus presented the highest diversity richness in soils. Fusarium and Penicillium have been isolated frequently in agro ecosystems including coffee plantations 23. Within the genus Fusarium are phytopathogenic species that cause diseases in coffee i.e. rot rotting, leaf spotting and death of plants 24. It is important to note that despite the prevalence of this fungi, there were no major problems of diseases caused by Fusarium on coffee plants in the farms visited which leads to the belief that a balance exists in all habitats between fungal community and parasites that impede their pathological expression. The most prevalent organisms in Kirinyaga central was Penicilium with 35% prevalence and the least prevalent organism being Alternaria with 3%. This indicates that Penicillium thrives in all the agro- ecologicalnzone under this study. This is also indicated in the findings where Penicillium was also the prevalent fungi in Kirinya West at 27% and in Mwea East at 22%. It inhabits the soil in greater proportion as compares to the surface of the berries. This is shown from the study where Penicillium was present in different proportions in soil samples obtained from all the sub-counties unlike its presence in only three sub-counties i.e Mwea East, Kirinyaga East and Kirinyaga West obtained from coffee berry surfaces. The findings of this study are consistent with a study that revealed Aspergillus and Penicillium as the major fungi from the soil of agricultural fields at Salur Manda, India 15. The study also indicated that Alternaria is a less prevalent fungi in all the agro-ecological zones of Kirinyaga County. It is seen to be less prevalent in both soil and coffee surfaces. This could be as a result of long-term continuous cropping system , a similar finding reported by Xiang 25. The study also showed Fusarium was prevalent in both soil and berries in all the zones with most prevalence reportedly in berries as compared to soil.
The results of this study also indicate that Trichoderma was less prevalent in all the agro-ecological zones. Trichoderma is being characterized by competitive saprophytic ability, which relates to its potential for producing a large numbers of spores that germinate rapidly and colonize any type of substrate 26. In this study, all coffee farms had a low prevalence of Trichoderma. A finding which differs from the study conducted by Lockwood 26 who found a greater prevalence in Trichoderma in a study on fungal diversity in a coffee plantation in Mexico. Some Trichoderma species are used in agriculture e.g. T. Hazarium which has been used proven potential as a growth promoter in various crops coffee. This species produces substances that act as catalyzers or accelerators of the primary meristematic tissue in the young parts of the plants 27. It has been shown that various strains have the capacity to accelerate the germination of coffee 28. Its presence in coffee plantations is thus very beneficial. The minimal prevalence and absence of these fungi in most of the farms across all the different zones could reflect as one of the factors that affect productivity of coffee in Kirinyaga. While Colletotrichum was reported to be rich in Berries, It was not found to dominate in soil across all the regions. This is due to specificity of the habitat of the fungi. The contrast between the high richness of species of the genus Penicillium and the low prevalence of Trichoderma in these farms where pesticides and fungicides are majorly used to control diseases, demonstrates the complexity of species responses in habitats. This arises the need for detailed study of the autecology of soil micro fungi. The low proportion of similarity demonstrated by less diverse fungi among the different agro-ecological zones suggests that the management practices and structure causes a substantial difference in species composition. A similar finding by Lockwood 26 whose study demonstrated low existence of diversity of similar species by low clustering patterns exhibited.
Whereas soils in Kirinyaga Central are slightly less rich in diversity of fungi as compared to the remaining three sub-counties, it has the most diverse fungi on the coffee berry surface after Kirinyaga East. Mwea East had the least diverse fungi on coffee berries surfaces. This could be attributed to the fact that a few farms were sampled in the region.
Conclusion
Although molecular methods continue to improve and become more rapidly available, microscopy and culture morphology remain commonly used and essential tools for the identification of fungi which has been used for identification of the 10 fungi isolated in this study. The results of the study have revealed low existence of species richness in the ecosystem. With an increased understanding of fungal community ecology, it is possible to develop strategies to conserve and use native micro fungal species in order to contribute to the organic management of coffee plantations and allow producers to get better processes on the international market. The study has also shown that the fungal community of moderate diversity is probably in relation to the cultural practices in Kirinyaga sub-counties. The isolation and identification of filamentous fungi from soils and berries from coffee farms in Kirinyaga County have further displayed a low prevalence of some economically important fungi e.g Trichoderma. This study recommends further work to further disentangle the triangle relationships between soil characteristics and potentially beneficial micro biota. The study has also revealed that soils in Kirinyaga Central are less rich in diversity as compared to the other sub-counties; this could be attributed to the climatic condition of the area. This study gives a valuable avenue for development of sustainable agricultural measures to improve microbiota activity promoting coffee growth in soils subjected to continous monoculture cropping and chemical applications for controlling coffee diseases.
Acknowledgment
The authors extend sincere appreciation to the National Research Fund for funding this research. The coffee farmers and Kirinyaga County Government are thanked for their cooperation and support during the field work. Sincere gratitude to the staff and management of Coffee Research Institute (CRI) for their support. This paper is published with the permission of the Director-General Kenya Agricultural & Livestock Research Organization through Institute Director, CRI, Ruiru, Kenya.
Conflict of Interest
The authors have not declared any conflict of interest.
References
- Lashermes, P., Combes, M.C., Ansaldi, C., Gichuru, E. and Noir, S., “Analysis of alien introgression in coffee tree (Coffea arabica L.)” Mol. Breed. 27: 223–232 (2011).
CrossRef - Vega, F. E., Simpkins, A., Aime, M. C., Posada, F., Peterson, S. W., Rehner, S. A., and Arnold, A. E., “Fungal endophyte diversity in coffee plants from Colombia, Hawai’i, Mexico and Puerto Ric,” fungal ecology, 3(3), 122-138 (2010).
CrossRef - Moser, C. O., & McIlwaine, C., “Encounters with violence in Latin America: urban poor perceptions from Columbia and Guatemala,” Psychology Press (2004).
CrossRef - Lewin, B., Giovannucci, D., & Varangis, P., “Coffee markets: new paradigms in global supply and demand.” World Bank Agriculture and Rural Development Discussion Paper, (3) (2004).
CrossRef - Babova, O., Occhipinti, A., & Maffei, M. E., “Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin,” Phytochemistry, 123, 33-39 (2016).
CrossRef - Thuku, G. K., & Gachanja, P., “Effects of reforms on productivity of coffee in Kenya,” International Journal of Business and Social Science, 4(15) (2013).
- Gathura, M. N., “Factors affecting small-scale coffee production in Githunguri District, Kenya,” International Journal of Academic Research in Business and Social Sciences, 3(9), 132 (2013).
CrossRef - CGK (County Government of Kirinyaga) Kirinyaga County. “Kirinyaga Rising” http://kirinyaga.go.ke/governor-waiguru-calls-for-guaranteed-coffee-prices-for-coffee-farmers/ Accessed May 12, 2021
- Royal News “ Kenya Coffee” https://en.wikipedia.org/wiki/Coffee_production_in_Kenya Accessed May 13, 2021
- KNA (Kenya News Agency). “Organic Farming Earns Kirinyaga Farmer A Fortune” https://www.kenyanews.go.ke/organic-farming-earns-kirinyaga-farmer-a-fortune/ Accessed May 13, 2021
- The Standard “Coffee berry disease ravages crop across Mt Kenya region” https://www.standardmedia.co.ke/business/article/2001283047/coffee-berry-disease-ravages-crop-across-mt-kenya-region#:~:text=The%20crop%20has%20been%20affected,loss%20of%20an%20entire%20crop.&text=The%20diseases%20have%20been%20reported,Murang’a%20and%20Nyeri%20counties. Accessed May 13, 2021
- Mahmood, I., Imadi, S. R., Shazadi, K., Gul, A., & Hakeem, K. R., “Effects of pesticides on environment”. In Plant, soil and microbes(pp. 253-269). Springer, Cham (2016).
CrossRef - EM (Energy Makers) “How does coffee affect the environment” https://energymakeovers.com.au/blog/how-does-coffee-affect-the-environment/#:~:text=Coffee%20processing%20plants%20often%20discharge,kill%20aquatic%20plants%20and%20animals.&text=As%20coffee%20demand%20grows%2C%20so,in%20come%20the%20new%20suppliers. Accessed May 13, 2021
- Turbé, A., De Toni, A., Benito, P., Lavelle, P., Lavelle, P., Camacho, N. R. and Mudgal, S., “ Soil biodiversity: functions, threats and tools for policy makers,” (2010).
- Gaddeyya, G., Niharika, P. S., Bharathi, P., and Kumar, P. R. Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Advances in Applied Science Research, 3(4), 2020-2026 (2012).
- Agarwal, M., Dheeman, S., Dubey, R. C., Kumar, P., Maheshwari, D. K., and Bajpai, V. K., “ Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench,” Microbiological research, 205, 40-47 (2017).
CrossRef - Locey, K. J., and Lennon, J. T., “Scaling laws predict global microbial diversit,” Proceedings of the National Academy of Sciences, 113(21), 5970-5975 (2016).
CrossRef - Blackwell, M., “The Fungi: 1, 2, 3… 5.1 million species?,” American Journal of Botany, 98(3), 426-438 (2011).
CrossRef - Ogolla, F. O., and Neema, D. Cultural., “ Morphological and Biochemical Identification of Xanthomonas Spp the Causative Agent of Bacteria Leaf Spot in Tomatoes in Wanguru, Mwea, Kirinyaga County, Keny.” International Journal of Research and Innovation in Applied Science, IV, 44-48 (2019).
- Serede, I. J., Mutua, B. M., and Raude, J. M., “Calibration of Channel Roughness Coefficient for Thiba Main Canal Reach in Mwea Irrigation Scheme, Kenya,” Hydrology, 3(6), 55 (2015).
CrossRef - Reddy, B. N., Saritha, K. V., and Hindumathi, A., “In vitro screening for antagonistic potential of seven species of Trichoderma against different plant pathogenic fungi.” J Biol, 2, 29-36 (2014).
- Watanabe., “T. Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to specie,”. CRC press (2010).
- Okoth, S NYONGESA B., Ayugi, V, Kang’ethe E., Korhonen, H and Joutsjoki V., “ Toxigenic potential of Aspergillus species occurring on maize kernels from two field-ecological zones in Kenya” Toxins, 4 (11) 991-1007 (2012).
CrossRef - Aoki, T., O’Donnell, K., and Geiser, D. M., “Systematics of key phytopathogenic Fusarium species: current status and future challenges,” Journal of General Plant Pathology, 80(3), 189-201(2014).
CrossRef - Sun, C., Li, F., Wei, M., Xiang, Z., Chen, C., and Xu, D., “Detection and Biological Characteristics of Alternaria alternata Resistant to Difenoconazole from Paris polyphylla var. chinensis, an Indigenous Medical Herb,” Plant Disease, PDIS-12 (2021).
CrossRef - Lockwood, J. L., “Fungistasis in soils” Biological Reviews, 52(1), 1-43.
CrossRef - Castro-Toro, AM, Rivillas-Osorio, CA, Villavicencio, B., and Chinchiná, C., “Bioregulation of Rhizoctonia solani in coffee germinators,” Cenicafé Technical Advances, 336, 1-8 . (1977) (2005).
- Delgado, Y., Cupull, R., Pérez, C., Sánchez, A., and Vilchez, M., “Effect of Azotobacter spp. in the stimulation of germination and posture development of Coffea arabica L,” Agricultural Center, 30(1), 26-31 (2003).


